Cloning and functional analysis of hypothalamic homeobox gene Bsx1a and its isoform, Bsx1b
- PMID: 17353277
- PMCID: PMC1899992
- DOI: 10.1128/MCB.01561-06
Cloning and functional analysis of hypothalamic homeobox gene Bsx1a and its isoform, Bsx1b
Abstract
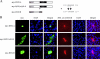
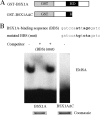
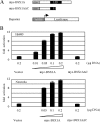
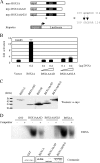
The hypothalamus is a key regulatory unit of the neuroendocrine system and plays an essential role in energy balance and reproduction. Despite its important role, the molecular mechanisms underlying hypothalamic development are not fully understood. Here, we report molecular analyses of a newly identified murine homeobox gene, Bsx/Bsx1a, that is expressed in the developing and postnatal hypothalamus. We demonstrate that BSX1A is a DNA binding protein and a transcriptional activator. Transcriptional reporter assays identified the C-terminal region of BSX1A as an activation domain. We have isolated an alternative splice form of Bsx1a, designated Bsx1b, which retains the N-terminal region but lacks the homeodomain. Analyses of subcellular localization using transfected cell lines revealed that BSX1A and BSX1B localize in the nuclei and cytoplasm, respectively. Immunohistochemical analyses suggested that both BSX1A and BSX1B are expressed in the neonatal hypothalamus. Taking these data together, we propose that alternative RNA splicing is involved in hypothalamic development/function.
Figures




Similar articles
-
REST is a key regulator in brain-specific homeobox gene expression during neuronal differentiation.J Neurochem. 2007 Dec;103(6):2565-74. doi: 10.1111/j.1471-4159.2007.04947.x. J Neurochem. 2007. PMID: 17944879
-
Two wobble-splicing events affect ING4 protein subnuclear localization and degradation.Exp Cell Res. 2008 Oct 15;314(17):3130-41. doi: 10.1016/j.yexcr.2008.08.002. Epub 2008 Aug 15. Exp Cell Res. 2008. PMID: 18775696
-
Conserved amino acid sequences confer nuclear localization upon the Prophet of Pit-1 pituitary transcription factor protein.Gene. 2004 Jul 21;336(2):263-73. doi: 10.1016/j.gene.2004.04.022. Gene. 2004. PMID: 15246537
-
Identification and characterization of a novel Delphilin variant with an alternative N-terminus.Brain Res Mol Brain Res. 2005 Nov 18;141(1):83-94. doi: 10.1016/j.molbrainres.2005.08.006. Epub 2005 Sep 15. Brain Res Mol Brain Res. 2005. PMID: 16168524
-
Expression of two protein isoforms of PAX7 is controlled by competing cleavage-polyadenylation and splicing.Gene. 2004 Nov 10;342(1):107-12. doi: 10.1016/j.gene.2004.07.030. Gene. 2004. PMID: 15527970
Cited by
-
Circadian regulation and molecular role of the Bsx homeobox gene in the adult pineal gland.J Pineal Res. 2020 Mar;68(2):e12629. doi: 10.1111/jpi.12629. Epub 2019 Dec 30. J Pineal Res. 2020. PMID: 31808568 Free PMC article.
-
The unique transcriptional activation domain of nuclear factor-I-X3 is critical to specifically induce marker gene expression in astrocytes.J Biol Chem. 2011 Mar 4;286(9):7315-26. doi: 10.1074/jbc.M110.152421. Epub 2010 Dec 28. J Biol Chem. 2011. PMID: 21189253 Free PMC article.
-
Characterization of a novel human HMBOX1 splicing variant lacking the homeodomain and with attenuated transcription repressor activity.Mol Biol Rep. 2010 Jul;37(6):2767-72. doi: 10.1007/s11033-009-9815-9. Epub 2009 Sep 15. Mol Biol Rep. 2010. PMID: 19757162
-
CuAS: a database of annotated transcripts generated by alternative splicing in cucumbers.BMC Plant Biol. 2020 Mar 18;20(1):119. doi: 10.1186/s12870-020-2312-y. BMC Plant Biol. 2020. PMID: 32183712 Free PMC article.
-
Genetic ablation of the Bsx homeodomain transcription factor in zebrafish: Impact on mature pineal gland morphology and circadian behavior.J Pineal Res. 2022 May;72(4):e12795. doi: 10.1111/jpi.12795. Epub 2022 Mar 31. J Pineal Res. 2022. PMID: 35249239 Free PMC article.
References
-
- Acampora, D., S. Mazan, F. Tuorto, V. Avantaggiato, J. L. Tremblay, D. Lazzaro, A. di Carlo, A. Mariano, P. E. Macchia, G. Corte, V. Macchia, J. Drouin, P. Brulet, and A. Simone. 1998. Transient dwarfism and hypogonadism in mice lacking Otx1 reveal prepubescent stage-specific control of pituitary levels of GH, FSH and LH. Development 125:1229-1239. - PubMed
-
- Ando, K., S. Shioda, H. Handa, and K. Kataoka. 2003. Isolation and characterization of an alternatively spliced variant of transcription factor Islet-1. J. Mol. Endocrinol. 31:419-425. - PubMed
-
- Cremona, M., E. Colombo, M. Andreazzoli, G. Cossu, and V. Broccoli. 2004. Bsx, an evolutionary conserved Brain Specific homeoboX gene expressed in the septum, epiphysis, mammillary bodies and arcuate nucleus. Gene Expr. Patterns 4:47-51. - PubMed
-
- Dasen, J. S., and M. G. Rosenfeld. 1999. Combinational codes in signaling and synergy: lessons from pituitary development. Curr. Opin. Gen. Dev. 9:566-574. - PubMed
-
- Dasen, J. S., and M. G. Rosenfeld. 1999. Signaling mechanisms in pituitary morphogenesis and cell fate determination. Curr. Opin. Cell Biol. 11:669-677. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
