The ChrA-ChrS and HrrA-HrrS signal transduction systems are required for activation of the hmuO promoter and repression of the hemA promoter in Corynebacterium diphtheriae
- PMID: 17353293
- PMCID: PMC1865786
- DOI: 10.1128/IAI.01821-06
The ChrA-ChrS and HrrA-HrrS signal transduction systems are required for activation of the hmuO promoter and repression of the hemA promoter in Corynebacterium diphtheriae
Abstract
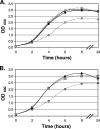
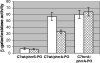
Transcription of the Corynebacterium diphtheriae hmuO gene, which encodes a heme oxygenase involved in heme iron utilization, is activated in a heme- or hemoglobin-dependent manner in part by the two-component system ChrA-ChrS. Mutation of either the chrA or the chrS gene resulted in a marked reduction of hemoglobin-dependent activation at the hmuO promoter in C. diphtheriae; however, it was observed that significant levels of hemoglobin-dependent expression were maintained in the mutants, suggesting that an additional activator is involved in regulation. A BLAST search of the C. diphtheriae genome sequence revealed a second two-component system, encoded by DIP2268 and DIP2267, that shares similarity with ChrS and ChrA, respectively; we have designated these genes hrrS (DIP2268) and hrrA (DIP2267). Analysis of hmuO promoter expression demonstrated that hemoglobin-dependent activity was fully abolished in strains from which both the chrA-chrS and the hrrA-hrrS two-component systems were deleted. Similarly, deletion of the sensor kinase genes chrS and hrrS or the genes encoding both of the response regulators chrA and hrrA also eliminated hemoglobin-dependent activation at the hmuO promoter. We also show that the regulators ChrA-ChrS and HrrA-HrrS are involved in the hemoglobin-dependent repression of the promoter upstream of hemA, which encodes a heme biosynthesis enzyme. Evidence for cross talk between the ChrA-ChrS and HrrA-HrrS systems is presented. In conclusion, these findings demonstrate that the ChrA-ChrS and HrrA-HrrS regulatory systems are critical for full hemoglobin-dependent activation at the hmuO promoter and also suggest that these two-component systems are involved in the complex mechanism of the regulation of heme homeostasis in C. diphtheriae.
Figures
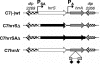


Similar articles
-
The ChrA response regulator in Corynebacterium diphtheriae controls hemin-regulated gene expression through binding to the hmuO and hrtAB promoter regions.J Bacteriol. 2012 Apr;194(7):1717-29. doi: 10.1128/JB.06801-11. Epub 2012 Jan 27. J Bacteriol. 2012. PMID: 22287525 Free PMC article.
-
Identification of a two-component signal transduction system from Corynebacterium diphtheriae that activates gene expression in response to the presence of heme and hemoglobin.J Bacteriol. 1999 Sep;181(17):5330-40. doi: 10.1128/JB.181.17.5330-5340.1999. J Bacteriol. 1999. PMID: 10464204 Free PMC article.
-
The ChrSA and HrrSA Two-Component Systems Are Required for Transcriptional Regulation of the hemA Promoter in Corynebacterium diphtheriae.J Bacteriol. 2016 Aug 25;198(18):2419-30. doi: 10.1128/JB.00339-16. Print 2016 Sep 15. J Bacteriol. 2016. PMID: 27381918 Free PMC article.
-
Regulation of heme oxygenase-1 gene transcription: recent advances and highlights from the International Conference (Uppsala, 2003) on Heme Oxygenase.Antioxid Redox Signal. 2004 Oct;6(5):924-33. doi: 10.1089/ars.2004.6.924. Antioxid Redox Signal. 2004. PMID: 15345152 Review.
-
Biology and molecular epidemiology of diphtheria toxin and the tox gene.J Infect Dis. 2000 Feb;181 Suppl 1:S156-67. doi: 10.1086/315554. J Infect Dis. 2000. PMID: 10657208 Review.
Cited by
-
Hemin binding protein C is found in outer membrane vesicles and protects Bartonella henselae against toxic concentrations of hemin.Infect Immun. 2012 Mar;80(3):929-42. doi: 10.1128/IAI.05769-11. Epub 2012 Jan 9. Infect Immun. 2012. PMID: 22232189 Free PMC article.
-
The ChrA response regulator in Corynebacterium diphtheriae controls hemin-regulated gene expression through binding to the hmuO and hrtAB promoter regions.J Bacteriol. 2012 Apr;194(7):1717-29. doi: 10.1128/JB.06801-11. Epub 2012 Jan 27. J Bacteriol. 2012. PMID: 22287525 Free PMC article.
-
The sbiTRS Operon Contributes to Stenobactin-Mediated Iron Utilization in Stenotrophomonas maltophilia.Microbiol Spectr. 2022 Dec 21;10(6):e0267322. doi: 10.1128/spectrum.02673-22. Epub 2022 Dec 1. Microbiol Spectr. 2022. PMID: 36453931 Free PMC article.
-
Two coregulated efflux transporters modulate intracellular heme and protoporphyrin IX availability in Streptococcus agalactiae.PLoS Pathog. 2010 Apr 22;6(4):e1000860. doi: 10.1371/journal.ppat.1000860. PLoS Pathog. 2010. PMID: 20421944 Free PMC article.
-
The ABC transporter HrtAB confers resistance to hemin toxicity and is regulated in a hemin-dependent manner by the ChrAS two-component system in Corynebacterium diphtheriae.J Bacteriol. 2010 Sep;192(18):4606-17. doi: 10.1128/JB.00525-10. Epub 2010 Jul 16. J Bacteriol. 2010. PMID: 20639324 Free PMC article.
References
-
- Baikalov, I., I. Schroder, M. Kaczor-Grzeskowiak, K. Grzeskowiak, R. P. Gunsalus, and R. E. Dickerson. 1996. Structure of the Escherichia coli response regulator NarL. Biochemistry 35:11053-110561. - PubMed
-
- Beale, S. I. 1996. Biosynthesis of hemes, p. 731-748. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. American Society for Microbiology Press, Washington, DC.
-
- Bibb, L. A., N. D. King, C. A. Kunkle, and M. P. Schmitt. 2005. Analysis of a heme-dependent signal transduction system in Corynebacterium diphtheriae: deletion of the chrAS genes results in heme sensitivity and diminished heme-dependent activation of the hmuO promoter. Infect. Immun. 73:7406-7412. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

