Treatment monitoring of brain creatine deficiency syndromes: a 1H- and 31P-MR spectroscopy study
- PMID: 17353334
- PMCID: PMC7977852
Treatment monitoring of brain creatine deficiency syndromes: a 1H- and 31P-MR spectroscopy study
Abstract
Background and purpose: Brain creatine (Cr) deficiencies (BCr-d) are rare disorders of creatine biosynthesis and transport. We performed consecutive measures of total Cr (tCr) and of its phosphorylated fraction, phosphocreatine (PCr), in the brains of children affected by Cr synthesis defects during a long period of therapy. The aim was to identify the optimal treatment strategy for these disorders.
Materials and methods: Two patients with guanidinoacetate methyltransferase defect (GAMT-d) were treated with different amounts of Cr and with diet restrictions aimed at reducing endogenous guanidinoacetate (GAA) synthesis. Three patients with arginine:glycine amidinotransferase defect (AGAT-d) were treated with different Cr intakes. The patients' treatments were monitored by means of (1)H- and (31)P-MR spectroscopy.
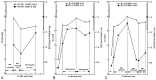
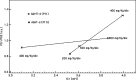
Results: Cr and PCr replenishment was lower in GAMT-d than in AGAT-d even when GAMT-d therapy was carried out with a very high Cr intake. Cr and especially PCr replenishment became more efficient only when GAA blood values were reduced. Adenosine triphosphate (ATP) was increased in the baseline phosphorous spectrum of GAMT-d, and it returned to a normal value with treatment. Brain pH and brain P(i) showed no significant change in the AGAT-d syndrome and at any Cr intake. However, 1 of the 2 GAMT-d patients manifested a lower brain pH level while consuming the GAA-lowering diet.
Conclusions: AGAT-d treatment needs lower Cr intake than GAMT-d. Cr supplementation in GAMT-d treatment should include diet restrictions aimed at reducing GAA concentration in body fluids. (1)H- and especially (31)P-MR spectroscopy are the ideal tools for monitoring the therapy response to these disorders.
Figures


Comment in
-
Is ATP elevated in patients with GAMT deficiency?AJNR Am J Neuroradiol. 2008 Feb;29(2):214; author reply 214. doi: 10.3174/ajnr.A0803. Epub 2007 Nov 16. AJNR Am J Neuroradiol. 2008. PMID: 18024580 Free PMC article. No abstract available.
References
-
- Stöckler S, Holzbach U, Hanefeld F, et al. Creatine deficiency in the brain: a new, treatable inborn error of metabolism. Pediatr Res 1994;36:409–13 - PubMed
-
- Battini R, Leuzzi V, Carducci CA, et al. Creatine depletion in a new case with AGAT deficiency: clinical and genetic study in a large pedigree. Mol Gen Metab 2002;77:326–31 - PubMed
-
- Stöckler S, Hanefeld F, Frahm J. Creatine replacement therapy in guanidinoacetate methyltransferase deficiency, a novel inborn error of metabolism. Lancet 1996;348:789–90 - PubMed
-
- Schulze A, Hess T, Wevers R, et al. Creatine deficiency syndrome caused by guanidinoacetate methyltransferase deficiency: diagnostic tools of a new inborn error of metabolism. J Pediatr 1997;131:626–31 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
