Chromosome breakage after G2 checkpoint release
- PMID: 17353355
- PMCID: PMC2064048
- DOI: 10.1083/jcb.200612047
Chromosome breakage after G2 checkpoint release
Abstract
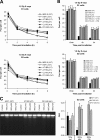
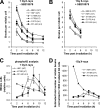
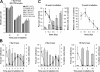
DNA double-strand break (DSB) repair and checkpoint control represent distinct mechanisms to reduce chromosomal instability. Ataxia telangiectasia (A-T) cells have checkpoint arrest and DSB repair defects. We examine the efficiency and interplay of ATM's G2 checkpoint and repair functions. Artemis cells manifest a repair defect identical and epistatic to A-T but show proficient checkpoint responses. Only a few G2 cells enter mitosis within 4 h after irradiation with 1 Gy but manifest multiple chromosome breaks. Most checkpoint-proficient cells arrest at the G2/M checkpoint, with the length of arrest being dependent on the repair capacity. Strikingly, cells released from checkpoint arrest display one to two chromosome breaks. This represents a major contribution to chromosome breakage. The presence of chromosome breaks in cells released from checkpoint arrest suggests that release occurs before the completion of DSB repair. Strikingly, we show that checkpoint release occurs at a point when approximately three to four premature chromosome condensation breaks and approximately 20 gammaH2AX foci remain.
Figures

References
-
- Asakawa, Y., and E. Gotoh. 1997. A method for detecting sister chromatid exchanges using prematurely condensed chromosomes and immunogold-silver staining. Mutagenesis. 12:175–177. - PubMed
-
- Cedervall, B., R. Wong, N. Albright, J. Dynlacht, P. Lambin, and W.C. Dewey. 1995. Methods for the quantification of DNA double-strand breaks determined from the distribution of DNA fragment sizes measured by pulsed-field gel electrophoresis. Radiat. Res. 143:8–16. - PubMed
-
- Cornforth, M.N., and J.S. Bedford. 1985. On the nature of a defect in cells from individuals with ataxia-telangiectasia. Science. 227:1589–1591. - PubMed
-
- Cornforth, M.N., and J.S. Bedford. 1993. Ionizing radiation damage and its early development in chromosomes. In Advances in Radiation Biology, vol. 17. J.T. Lett and W.K. Sinclair, editors. Academic Press, San Diego. 423–496.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

