A role for SC35 and hnRNPA1 in the determination of amyloid precursor protein isoforms
- PMID: 17353911
- PMCID: PMC2684093
- DOI: 10.1038/sj.mp.4001971
A role for SC35 and hnRNPA1 in the determination of amyloid precursor protein isoforms
Abstract
The beta-amyloid peptide (Abeta) that accumulates in senile plaques in Alzheimer's disease is formed by cleavage of the amyloid precursor protein (APP). The APP gene has several intronic Alu elements inserted in either the sense or antisense orientation. In this study, we demonstrate that binding of SC35 and hnRNPA1 to Alu elements on either side of exon 7 in the transcribed pre-mRNA is involved in alternative splicing of APP exons 7 and 8. Neuronal cells transfected with the full-length form of APP secrete higher levels of Abeta than cells transfected with the APP695 isoform lacking exons 7 and 8. Finally, we show that treatment of neuronal cells with estradiol results in increased expression of APP695, SC35 and hnRNPA1, and lowers the level of secreted Abeta. An understanding of the regulation of splicing of APP may lead to the identification of new targets for treating Alzheimer's disease.
Figures




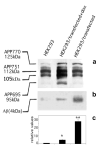
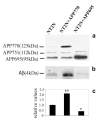

Similar articles
-
Neuronal ELAVL proteins utilize AUF-1 as a co-partner to induce neuron-specific alternative splicing of APP.Sci Rep. 2017 Mar 14;7:44507. doi: 10.1038/srep44507. Sci Rep. 2017. PMID: 28291226 Free PMC article.
-
Alternative splicing of the adenylyl cyclase stimulatory G-protein G alpha(s) is regulated by SF2/ASF and heterogeneous nuclear ribonucleoprotein A1 (hnRNPA1) and involves the use of an unusual TG 3'-splice Site.J Biol Chem. 2002 May 3;277(18):15241-51. doi: 10.1074/jbc.M109046200. Epub 2002 Feb 1. J Biol Chem. 2002. PMID: 11825891
-
Interaction of hnRNPA1/A2 and DAZAP1 with an Alu-derived intronic splicing enhancer regulates ATM aberrant splicing.PLoS One. 2011;6(8):e23349. doi: 10.1371/journal.pone.0023349. Epub 2011 Aug 8. PLoS One. 2011. PMID: 21858080 Free PMC article.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
Regulation of Alzheimer beta-amyloid precursor trafficking and metabolism.Biochim Biophys Acta. 2000 Jul 26;1502(1):44-52. doi: 10.1016/s0925-4439(00)00031-4. Biochim Biophys Acta. 2000. PMID: 10899430 Review.
Cited by
-
Alternative Splicing of the Pituitary Adenylate Cyclase-Activating Polypeptide Receptor PAC1: Mechanisms of Fine Tuning of Brain Activity.Front Endocrinol (Lausanne). 2013 May 21;4:55. doi: 10.3389/fendo.2013.00055. eCollection 2013. Front Endocrinol (Lausanne). 2013. PMID: 23734144 Free PMC article.
-
The involvement of microRNAs in neurodegenerative diseases.Front Cell Neurosci. 2013 Dec 19;7:265. doi: 10.3389/fncel.2013.00265. Front Cell Neurosci. 2013. PMID: 24391543 Free PMC article. Review.
-
hnRNP A1, hnRNP A2B1, and hnRNP K are dysregulated in tauopathies, but do not colocalize with tau pathology.Brain Pathol. 2025 May;35(3):e13305. doi: 10.1111/bpa.13305. Epub 2024 Oct 1. Brain Pathol. 2025. PMID: 39354671 Free PMC article.
-
Spliceosome protein alterations differentiate hubs of the default mode connectome during the progression of Alzheimer's disease.Brain Pathol. 2025 Sep;35(5):e70004. doi: 10.1111/bpa.70004. Epub 2025 Mar 23. Brain Pathol. 2025. PMID: 40122679 Free PMC article.
-
Oligomeric, phosphorylated, and truncated tau and spliceosome pathology within the entorhinal-hippocampal connectome across stages of Alzheimer's disease.J Comp Neurol. 2023 Dec;531(18):2080-2108. doi: 10.1002/cne.25466. Epub 2023 Mar 29. J Comp Neurol. 2023. PMID: 36989381 Free PMC article.
References
-
- Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. - PubMed
-
- Kang J, Lemaire H-G, Unterbeck A, Salbaum JM, Masters CL, Grzeschik K-H, et al. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733–736. - PubMed
-
- Goldgaber D, Lerman MI, McBride OW, Saffiotti U, Gajdusek DC. Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer’s disease. Science. 1987;235:877–880. - PubMed
-
- Tanzi RE, Gusella JF, Watkins PC, Bruns GA, StGeorge-Hyslop O, Van Keuren ML, et al. Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 1987;235:880–884. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

