Gyrase inhibitors induce an oxidative damage cellular death pathway in Escherichia coli
- PMID: 17353933
- PMCID: PMC1847949
- DOI: 10.1038/msb4100135
Gyrase inhibitors induce an oxidative damage cellular death pathway in Escherichia coli
Abstract
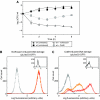
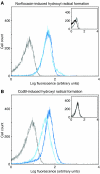
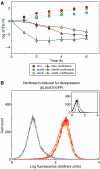
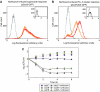
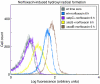
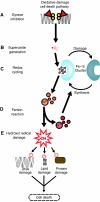
Modulation of bacterial chromosomal supercoiling is a function of DNA gyrase-catalyzed strand breakage and rejoining. This reaction is exploited by both antibiotic and proteic gyrase inhibitors, which trap the gyrase molecule at the DNA cleavage stage. Owing to this interaction, double-stranded DNA breaks are introduced and replication machinery is arrested at blocked replication forks. This immediately results in bacteriostasis and ultimately induces cell death. Here we demonstrate, through a series of phenotypic and gene expression analyses, that superoxide and hydroxyl radical oxidative species are generated following gyrase poisoning and play an important role in cell killing by gyrase inhibitors. We show that superoxide-mediated oxidation of iron-sulfur clusters promotes a breakdown of iron regulatory dynamics; in turn, iron misregulation drives the generation of highly destructive hydroxyl radicals via the Fenton reaction. Importantly, our data reveal that blockage of hydroxyl radical formation increases the survival of gyrase-poisoned cells. Together, this series of biochemical reactions appears to compose a maladaptive response, that serves to amplify the primary effect of gyrase inhibition by oxidatively damaging DNA, proteins and lipids.
Figures


Comment in
-
The complexities of antibiotic action.Mol Syst Biol. 2007;3:142. doi: 10.1038/msb4100184. Epub 2007 Oct 16. Mol Syst Biol. 2007. PMID: 17940532 Free PMC article. No abstract available.
References
-
- Andrews SC, Robinson AK, Rodriguez-Quinones F (2003) Bacterial iron homeostasis. FEMS Microbiol Rev 27: 215–237 - PubMed
-
- Aruoma OI, Halliwell B, Dizdaroglu M (1989) Iron ion-dependent modification of bases in DNA by the superoxide radical-generating system hypoxanthine/xanthine oxidase. J Biol Chem 264: 13024–13028 - PubMed
-
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G (2000) Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 25: 25–29 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

