Diminished forkhead box P3/CD25 double-positive T regulatory cells are associated with the increased nuclear factor-kappaB ligand (RANKL+) T cells in bone resorption lesion of periodontal disease
- PMID: 17355249
- PMCID: PMC1868884
- DOI: 10.1111/j.1365-2249.2006.03318.x
Diminished forkhead box P3/CD25 double-positive T regulatory cells are associated with the increased nuclear factor-kappaB ligand (RANKL+) T cells in bone resorption lesion of periodontal disease
Abstract
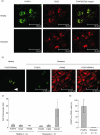
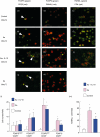
Periodontal disease involves multi-bacterial infections accompanied by inflammatory bone resorption lesions. The abundant T and B lymphocyte infiltrates are the major sources of the osteoclast differentiation factor, receptor activator for nuclear factor-kappaB ligand (RANKL) which, in turn, contributes to the development of bone resorption in periodontal disease. In the present study, we found that the concentrations of RANKL and regulatory T cell (T(reg))-associated cytokine, interleukin (IL)-10, in the periodontal tissue homogenates were correlated negatively, whereas RANKL and proinflammatory cytokine, IL-1beta, showed positive correlation. Also, according to the fluorescent-immunohistochemistry, the frequency of forkhead box P3 (FoxP3)/CD25 double-positive cells was diminished strikingly in the bone resorption lesion of periodontal disease compared to healthy gingival tissue, while CD25 or FoxP3 single positive cells were still observed in lesions where abundant RANKL+ lymphocytes were present. Very importantly, few or no expressions of FoxP3 by the RANKL+ lymphocytes were observed in the diseased periodontal tissues. Finally, IL-10 suppressed both soluble RANKL (sRANKL) and membrane RANKL (mRANKL) expression by peripheral blood mononuclear cells (PBMC) activated in vitro in a bacterial antigen-specific manner. Taken together, these results suggested that FoxP3/CD25 double-positive T(reg) cells may play a role in the down-regulation of RANKL expression by activated lymphocytes in periodontal diseased tissues. This leads to the conclusion that the phenomenon of diminished CD25+FoxP3+ T(reg) cells appears to be associated with the increased RANKL+ T cells in the bone resorption lesion of periodontal disease.
Figures

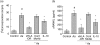
Similar articles
-
Over-expression of forkhead box P3 and its association with receptor activator of nuclear factor-kappa B ligand, interleukin (IL) -17, IL-10 and transforming growth factor-beta during the progression of chronic periodontitis.J Clin Periodontol. 2009 May;36(5):396-403. doi: 10.1111/j.1600-051X.2009.01390.x. J Clin Periodontol. 2009. PMID: 19419438
-
B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease.Am J Pathol. 2006 Sep;169(3):987-98. doi: 10.2353/ajpath.2006.060180. Am J Pathol. 2006. PMID: 16936272 Free PMC article.
-
Forkhead box P3-positive regulatory T-cells and interleukin 17-positive T-helper 17 cells in chronic inflammatory periodontal disease.J Periodontal Res. 2014 Dec;49(6):817-26. doi: 10.1111/jre.12169. Epub 2014 Feb 8. J Periodontal Res. 2014. PMID: 24506561
-
Immune response: the key to bone resorption in periodontal disease.J Periodontol. 2005 Nov;76(11 Suppl):2033-41. doi: 10.1902/jop.2005.76.11-S.2033. J Periodontol. 2005. PMID: 16277573 Review.
-
Potassium channel-blockers as therapeutic agents to interfere with bone resorption of periodontal disease.J Dent Res. 2005 Jun;84(6):488-99. doi: 10.1177/154405910508400603. J Dent Res. 2005. PMID: 15914584 Review.
Cited by
-
Estrogen enhances the functions of CD4(+)CD25(+)Foxp3(+) regulatory T cells that suppress osteoclast differentiation and bone resorption in vitro.Cell Mol Immunol. 2011 Jan;8(1):50-8. doi: 10.1038/cmi.2010.54. Epub 2010 Dec 6. Cell Mol Immunol. 2011. PMID: 21200384 Free PMC article.
-
CD8+ T Cells in Chronic Periodontitis: Roles and Rules.Front Immunol. 2017 Feb 21;8:145. doi: 10.3389/fimmu.2017.00145. eCollection 2017. Front Immunol. 2017. PMID: 28270813 Free PMC article. No abstract available.
-
Association between Porphyromonas Gingivalis and systemic diseases: Focus on T cells-mediated adaptive immunity.Front Cell Infect Microbiol. 2022 Nov 17;12:1026457. doi: 10.3389/fcimb.2022.1026457. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36467726 Free PMC article. Review.
-
Expression profile of IL-35 mRNA in gingiva of chronic periodontitis and aggressive periodontitis patients: a semiquantitative RT-PCR study.Dis Markers. 2013;35(6):819-23. doi: 10.1155/2013/489648. Epub 2013 Nov 27. Dis Markers. 2013. PMID: 24376289 Free PMC article.
-
Inflammation indices in association with periodontitis and cancer.Periodontol 2000. 2024 Oct;96(1):281-315. doi: 10.1111/prd.12612. Epub 2024 Sep 24. Periodontol 2000. 2024. PMID: 39317462 Free PMC article. Review.
References
-
- Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–52. - PubMed
-
- Nakajima T, Ueki-Maruyama K, Oda T, et al. Regulatory T-cells infiltrate periodontal disease tissues. J Dent Res. 2005;84:639–43. - PubMed
-
- Horwood NJ, Kartsogiannis V, Quinn JM, Romas E, Martin TJ, Gillespie MT. Activated T lymphocytes support osteoclast formation in vitro. Biochem Biophys Res Commun. 1999;265:144–50. - PubMed
-
- Kong YY, Feige U, Sarosi I, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–9. - PubMed
-
- Valverde P, Kawai T, Taubman MA. Selective blockade of voltage-gated potassium channels reduces inflammatory bone resorption in experimental periodontal disease. J Bone Miner Res. 2004;19:155–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

