ADAMs in cancer cell proliferation and progression
- PMID: 17355265
- PMCID: PMC11160018
- DOI: 10.1111/j.1349-7006.2007.00434.x
ADAMs in cancer cell proliferation and progression
Abstract
A disintegrin and metalloproteinases (ADAMs) are a new gene family of proteins with sequence similarity to the reprolysin family of snake venomases that share the metalloproteinase domain with matrix metalloproteinases (MMPs). They are structurally classified into two groups: the membrane-anchored ADAM and ADAM with thrombospondin motifs (ADAMTS). These molecules are involved in various biological events such as cell adhesion, cell fusion, cell migration, membrane protein shedding and proteolysis. Studies on the biochemical characteristics and biological functions of ADAMs are in progress, and accumulated lines of evidence have shown that some ADAMs are expressed in malignant tumors and participate in the pathology of cancers. The activities of ADAMs are regulated by gene expression, intracytoplasmic and pericellular regulation, activation of the zymogens and inhibition of activities by inhibitors. Many ADAM species, including ADAM8, ADAM9, ADAM10, ADAM12, ADAM15, ADAM17, ADAM19, ADAM28, ADAMTS1, ADAMTS4 and ADAMTS5, are expressed in human malignant tumors. Many of them are involved in the regulation of growth factor activities and integrin functions, leading to promotion of cell growth and invasion, although the precise mechanisms of these are not clear at the present time. In this article, we review recent information about ADAM family members and their implications for cancer cell proliferation and progression.
Figures

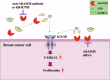
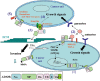
References
-
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2002; 2: 161–74. - PubMed
-
- Shiomi T, Okada Y. MT1‐MMP and MMP‐7 in invasion and metastasis of human cancers. Cancer Metastasis Rev 2003; 22: 145–52. - PubMed
-
- Seals DF, Courtneidge SA. The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes Dev 2003; 17: 7–30. - PubMed
-
- Gaultier A, Cousin H, Darribere T, Alfandari D. ADAM13 disintegrin and cysteine‐rich domains bind to the second heparin‐binding domain of fibronectin. J Biol Chem 2000; 277: 23 336–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

