Glycoprotein structural genomics: solving the glycosylation problem
- PMID: 17355862
- PMCID: PMC1885966
- DOI: 10.1016/j.str.2007.01.011
Glycoprotein structural genomics: solving the glycosylation problem
Abstract
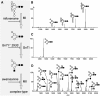
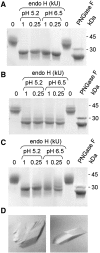
Glycoproteins present special problems for structural genomic analysis because they often require glycosylation in order to fold correctly, whereas their chemical and conformational heterogeneity generally inhibits crystallization. We show that the "glycosylation problem" can be solved by expressing glycoproteins transiently in mammalian cells in the presence of the N-glycosylation processing inhibitors, kifunensine or swainsonine. This allows the correct folding of the glycoproteins, but leaves them sensitive to enzymes, such as endoglycosidase H, that reduce the N-glycans to single residues, enhancing crystallization. Since the scalability of transient mammalian expression is now comparable to that of bacterial systems, this approach should relieve one of the major bottlenecks in structural genomic analysis.
Figures
References
-
- Aricescu A.R., Lu W., Jones E.Y. A time and cost efficient system for high level protein production in mammalian cells. Acta Crystallogr. D Biol. Crystallogr. 2006;10:1243–1250. - PubMed
-
- Bebbington C., Hentschell C. The use of vectors based on gene amplification for the expression of cloned genes in mammalian cells. In: Glover D.M., editor. DNA Cloning III: A Practical Approach. IRL Press; Oxford, UK: 1987. pp. 163–188.
-
- Berntzen G., Lunde E., Flobakk M., Andersen J.T., Lauvrak V., Sandlie I. Prolonged and increased expression of soluble Fc receptors, IgG and a TCR-Ig fusion protein by transiently transfected adherent 293E cells. J. Immunol. Methods. 2005;298:93–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

