Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma
- PMID: 17356056
- PMCID: PMC2094295
- DOI: 10.1136/thx.2006.073429
Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma
Abstract
Background: Non-eosinophilic asthma is a potentially important clinicopathological phenotype since there is evidence that it responds poorly to inhaled corticosteroid therapy. However, little is known about the underlying airway immunopathology and there are no data from placebo-controlled studies examining the effect of inhaled corticosteroids.
Methods: Airway immunopathology was investigated using induced sputum, bronchial biopsies, bronchial wash and bronchoalveolar lavage in 12 patients with symptomatic eosinophilic asthma, 11 patients with non-eosinophilic asthma and 10 healthy controls. The patients with non-eosinophilic asthma and 6 different patients with eosinophilic asthma entered a randomised, double-blind, placebo-controlled crossover study in which the effects of inhaled mometasone 400 microg once daily for 8 weeks on airway responsiveness and asthma quality of life were investigated.
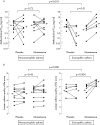
Results: Patients with non-eosinophilic asthma had absence of eosinophils in the mucosa (median 4.4 cells/mm(2) vs 23 cells/mm(2) in eosinophilic asthma and 0 cells/mm(2) in normal controls; p = 0.03) and normal subepithelial layer thickness (5.8 microm vs 10.3 microm in eosinophilic asthma and 5.1 microm in controls, p = 0.002). Non-eosinophilic and eosinophilic asthma groups had increased mast cell numbers in the airway smooth muscle compared with normal controls (9 vs 8 vs 0 cells/mm(2), p = 0.016). Compared with placebo, 8 weeks of treatment with inhaled mometasone led to less improvement in methacholine PC(20) (0.5 vs 5.5 doubling concentrations, 95% CI of difference 1.1 to 9.1; p = 0.018) and asthma quality of life (0.2 vs 1.0 points, 95% CI of difference 0.27 to 1.43; p = 0.008).
Conclusions: Non-eosinophilic asthma represents a pathologically distinct disease phenotype which is characterised by the absence of airway eosinophilia, normal subepithelial layer thickness and a poor short-term response to treatment with inhaled corticosteroids.
Conflict of interest statement
Competing interests: None.
Comment in
-
What do non-eosinophilic asthma and airway remodelling tell us about persistent asthma?Thorax. 2007 Dec;62(12):1034-6. doi: 10.1136/thx.2007.079061. Thorax. 2007. PMID: 18025139 Free PMC article.
References
-
- Rackemann F M. A clinical classification of asthma. Am J Med Sci 1921CLXII802
-
- Aas K. Heterogeneity of bronchial asthma. Sub‐populations—or different stages of the disease. Allergy 1981363–14. - PubMed
-
- Humbert M, Menz G, Ying S.et al The immunopathology of extrinsic (atopic) and intrinsic (non‐atopic) asthma: more similarities than differences. Immunol Today 199920528–533. - PubMed
-
- Humbert M, Durham S R, Ying S.et al IL‐4 and IL‐5 mRNA and protein in bronchial biopsies from patients with atopic and nonatopic asthma: evidence against “intrinsic” asthma being a distinct immunopathologic entity. Am J Respir Crit Care Med 19961541497–1504. - PubMed
-
- Pavord I D, Brightling C E, Woltmann G.et al Non‐eosinophilic corticosteroid unresponsive asthma. Lancet 19993532213–2214. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical