Precancerous stem cells have the potential for both benign and malignant differentiation
- PMID: 17356702
- PMCID: PMC1808425
- DOI: 10.1371/journal.pone.0000293
Precancerous stem cells have the potential for both benign and malignant differentiation
Abstract
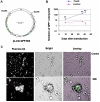
Cancer stem cells (CSCs) have been identified in hematopoietic and solid tumors. However, their precursors-namely, precancerous stem cells (pCSCs) -have not been characterized. Here we experimentally define the pCSCs that have the potential for both benign and malignant differentiation, depending on environmental cues. While clonal pCSCs can develop into various types of tissue cells in immunocompetent mice without developing into cancer, they often develop, however, into leukemic or solid cancers composed of various types of cancer cells in immunodeficient mice. The progress of the pCSCs to cancers is associated with the up-regulation of c-kit and Sca-1, as well as with lineage markers. Mechanistically, the pCSCs are regulated by the PIWI/AGO family gene called piwil2. Our results provide clear evidence that a single clone of pCSCs has the potential for both benign and malignant differentiation, depending on the environmental cues. We anticipate pCSCs to be a novel target for the early detection, prevention, and therapy of cancers.
Conflict of interest statement
Figures
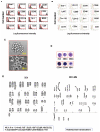

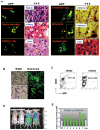
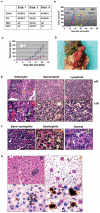

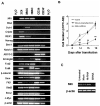

References
-
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem Cells, Cancer, and Cancer Stem Cells. Nature. 2001;414:105–11. - PubMed
-
- Wang JC, Dick JE. Cancer Stem Cells: Lessons from Leukemia. Trends Cell Biol. 2005;15:494–501. - PubMed
-
- Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, et al. Cancer Stem Cells–Perspectives on Current Status and Future Directions: Aacr Workshop on Cancer Stem Cells. Cancer Res. 2006;66:9339–44. - PubMed
-
- Bonnet D, Dick JE. Human Acute Myeloid Leukemia Is Organized as a Hierarchy That Originates from a Primitive Hematopoietic Cell. Nat Med. 1997;3:730–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

