House dust mite allergen avoidance and self-management in allergic patients with asthma: randomised controlled trial
- PMID: 17359604
- PMCID: PMC2042544
House dust mite allergen avoidance and self-management in allergic patients with asthma: randomised controlled trial
Abstract
Background: The efficacy of bed covers that are impermeable to house dust mites has been disputed.
Aim: The aim of the present study was to investigate whether the combination of 'house dust mite impermeable' covers and a self-management plan, based on peak flow values and symptoms, leads to reduced use of inhaled corticosteroids (ICS) than self-management alone.
Design of study: Prospective, randomised, double blind, placebo-controlled trial.
Setting: Primary care in a south-eastern region of the Netherlands.
Method: Asthma patients aged between 16 and 60 years with a house dust mite allergy requiring ICS were randomised to intervention and placebo groups. They were trained to use a self-management plan based on peak flow and symptoms. After a 3-month training period, the intervention commenced using house dust mite impermeable and placebo bed covers. The follow-up period was 2 years. Primary outcome was the use of ICS; secondary outcomes were peak expiratory flow parameters, asthma control, and symptoms.
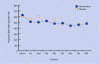
Results: One hundred and twenty-six patients started the intervention with house dust mite impermeable or placebo bed covers. After 1 and 2 years, significant differences in allergen exposure were found between the intervention and control groups (P<0.001). No significant difference between the intervention and control groups was found in the dose of ICS (P = 0.08), morning peak flow (P = 0.52), peak flow variability (P = 0.36), dyspnoea (P = 0.46), wheezing (P = 0.77), or coughing (P = 0.41). There was no difference in asthma control between the intervention and control groups.
Conclusion: House dust mite impermeable bed covers combined with self-management do not lead to reduced use of ICS compared with self-management alone.
Figures
References
-
- Djukanovic R, Roche WR, Wilson JW, et al. Mucosal inflammation in asthma. Am Rev Respir Dis. 1990;142(2):434–457. - PubMed
-
- Juniper EF, Kline PA, Vanzieleghem MA, et al. Effect of long-term treatment with an inhaled corticosteroid (budesonide) on airway hyperresponsiveness and clinical asthma in nonsteroid-dependent asthmatics. Am Rev Respir Dis. 1990;142(4):832–836. - PubMed
-
- Haahtela T, Jarvinen M, Kava T, et al. Comparison of a beta 2-agonist, terbutaline, with an inhaled corticosteroid, budesonide, in newly detected asthma. N Engl J Med. 1991;325(6):388–392. - PubMed
-
- Kerstjens HA, Brand PL, Hughes MD, et al. A comparison of bronchodilator therapy with or without inhaled corticosteroid therapy for obstructive airways disease. Dutch Chronic Non-Specific Lung Disease Study Group. N Engl J Med. 1992;327(20):1413–1419. - PubMed
-
- Dompeling E, van Schayck CP, van Grunsven PM, et al. Slowing the deterioration of asthma and chronic obstructive pulmonary disease observed during bronchodilator therapy by adding inhaled corticosteroids. A 4-year prospective study. Ann Intern Med. 1993;118(10):770–778. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials