A dominant antigenic epitope on SARS-CoV spike protein identified by an avian single-chain variable fragment (scFv)-expressing phage
- PMID: 17360045
- PMCID: PMC7112517
- DOI: 10.1016/j.vetimm.2007.02.001
A dominant antigenic epitope on SARS-CoV spike protein identified by an avian single-chain variable fragment (scFv)-expressing phage
Abstract
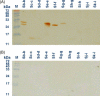
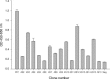
Severe acute respiratory syndrome (SARS) is a newly emergent human disease, which requires rapid diagnosis and effective therapy. Among antibody sources, immunoglobulin Y (IgY) is the major antibody found in chicken eggs and can be used as an alternative to mammalian antibodies normally used in research and immunotherapy. In this study, phage-expressing chicken monoclonal scFv antibody was chosen and characterized with phage display antibody technology. Truncated fragments of SARS-CoV spike protein were cloned in pET-21 vector and expressed in BL-21 Escherichia coli (E. coli) cells. After purification, the purity of these recombinant spike proteins was examined on SDS-PAGE and their identity verified with Western blot analysis using anti-his antibodies and sera from convalescent stage SARS-CoV-infected patients. Using these bacteria-derived proteins to immunize chickens, it was found that polyclonal IgY antibodies in the egg yolk and sera were highly reactive to the immunogens, as shown by Western blot and immunocytochemical staining analysis. A phage displaying scFv library was also established from spleen B cells of immunized chicken with 5 x 10(7) clones. After four panning cycles, the eluted phage titer showed a 10-fold increase. In sequence analysis with chicken germline gene, five phage clones reacted, with large dissimilarities of between 31 and 62%, in the complementarity-determining regions, one dominant phage 4S1 had strong binding to fragment Se-e, located between amino acid residues 456-650 of the spike protein and this particular phage had significantly strong binding to SARS-CoV-infected Vero E6 cells. Based on the results, we conclude that generating specific scFv-expressing phage binders with the phage display system can be successfully achieved and that this knowledge can be applied in clinical or academic research.
Figures



Similar articles
-
Chicken single-chain variable fragments against the SARS-CoV spike protein.J Virol Methods. 2007 Dec;146(1-2):104-11. doi: 10.1016/j.jviromet.2007.06.010. Epub 2007 Jul 23. J Virol Methods. 2007. PMID: 17643500 Free PMC article.
-
Human monoclonal antibody combination against SARS coronavirus: synergy and coverage of escape mutants.PLoS Med. 2006 Jul;3(7):e237. doi: 10.1371/journal.pmed.0030237. PLoS Med. 2006. PMID: 16796401 Free PMC article.
-
SARS patients-derived human recombinant antibodies to S and M proteins efficiently neutralize SARS-coronavirus infectivity.Biomed Environ Sci. 2005 Dec;18(6):363-74. Biomed Environ Sci. 2005. PMID: 16544518
-
An approach towards development of monoclonal IgY antibodies against SARS CoV-2 spike protein (S) using phage display method: A review.Int Immunopharmacol. 2020 Aug;85:106654. doi: 10.1016/j.intimp.2020.106654. Epub 2020 Jun 3. Int Immunopharmacol. 2020. PMID: 32512271 Free PMC article. Review.
-
Severe acute respiratory syndrome (SARS): development of diagnostics and antivirals.Ann N Y Acad Sci. 2006 May;1067(1):500-5. doi: 10.1196/annals.1354.072. Ann N Y Acad Sci. 2006. PMID: 16804033 Free PMC article. Review.
Cited by
-
Phage Display: A Powerful Technology for the Generation of High-Specificity Affinity Reagents from Alternative Immune Sources.Methods Mol Biol. 2017;1485:85-99. doi: 10.1007/978-1-4939-6412-3_6. Methods Mol Biol. 2017. PMID: 27730550 Free PMC article.
-
Isolation, characterization, and molecular modeling of a rheumatoid factor from a Hepatitis C virus infected patient with Sjögren's syndrome.ScientificWorldJournal. 2013 Dec 30;2013:516516. doi: 10.1155/2013/516516. eCollection 2013. ScientificWorldJournal. 2013. PMID: 24489505 Free PMC article.
-
Economically Feasible Mass Production of Egg Yolk Powder Tablets (Chicken IgY) for Global COVID-19 Transmission Prevention.Res Sq [Preprint]. 2025 Mar 19:rs.3.rs-5068132. doi: 10.21203/rs.3.rs-5068132/v1. Res Sq. 2025. PMID: 40166022 Free PMC article. Preprint.
-
Generation of Chicken IgY against SARS-COV-2 Spike Protein and Epitope Mapping.J Immunol Res. 2020 Oct 17;2020:9465398. doi: 10.1155/2020/9465398. eCollection 2020. J Immunol Res. 2020. PMID: 33134398 Free PMC article.
-
Generation and characterization of anti-alpha-enolase single-chain antibodies in chicken.Vet Immunol Immunopathol. 2010 Oct 15;137(3-4):251-60. doi: 10.1016/j.vetimm.2010.06.001. Epub 2010 Jun 22. Vet Immunol Immunopathol. 2010. PMID: 20655599 Free PMC article.
References
-
- Abouzid K., Ndeboko B., Durantel S., Jamard C., Zoulim F., Buronfosse T., Cova L. Genetic vaccination for production of DNA-designed antibodies specific to Hepadnavirus envelope proteins. Vaccine. 2005;24:4615–4617. - PubMed
-
- Akita E.M., Nakai S. Comparison of four purification methods for the production of immunoglobulins from eggs laid by hens immunized with an enterotoxigenic E. coli strain. J. Immunol. Methods. 1993;160:207–214. - PubMed
-
- Akita E.M., Nakai S. Production and purification of Fab’ fragments from chicken egg yolk immunoglobulin Y (IgY) J. Immunol. Methods. 1993;162:155–164. - PubMed
-
- Andris-Widhopf J., Rader C., Steinberger P., Fuller R., Barbas C.F., III Methods for the generation of chicken monoclonal antibody fragments by phage display. J. Immunol. Methods. 2000;242:159–181. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

