Quorum-sensing blockade as a strategy for enhancing host defences against bacterial pathogens
- PMID: 17360273
- PMCID: PMC2435584
- DOI: 10.1098/rstb.2007.2046
Quorum-sensing blockade as a strategy for enhancing host defences against bacterial pathogens
Abstract
Conventional antibiotics target the growth and the basal life processes of bacteria leading to growth arrest and cell death. The selective force that is inherently linked to this mode of action eventually selects out antibiotic-resistant variants. The most obvious alternative to antibiotic-mediated killing or growth inhibition would be to attenuate the bacteria with respect to pathogenicity. The realization that Pseudomonas aeruginosa, and a number of other pathogens, controls much of their virulence arsenal by means of extracellular signal molecules in a process denoted quorum sensing (QS) gave rise to a new 'drug target rush'. Recently, QS has been shown to be involved in the development of tolerance to various antimicrobial treatments and immune modulation. The regulation of virulence via QS confers a strategic advantage over host defences. Consequently, a drug capable of blocking QS is likely to increase the susceptibility of the infecting organism to host defences and its clearance from the host. The use of QS signal blockers to attenuate bacterial pathogenicity, rather than bacterial growth, is therefore highly attractive, particularly with respect to the emergence of multi-antibiotic resistant bacteria.
Figures
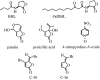


References
-
- Allesen-Holm M, Barken Bundvig K, Yang L, Klausen M, Webb J.S, Kjelleberg S, Molin S, Givskov M, Tolker-Nielsen T. A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol. Microbiol. 2005;54:1114–1128. - PubMed
-
- Ankri S, Mirelman D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999;1:125–129. doi:10.1016/S1286-4579(99)80003-3 - DOI - PubMed
-
- Anthony M, Kjelleberg S, Kumar N, de Nys R, Read R, Stainberg P. Reinvestigation of the sulfuric acid-catalysed cyclisation of brominated 2-alkylllevulinic acids to 3-alkyl-5-methylene-2(5H)-furanones. Tetrahedron. 1997;53:15 813–15 826. doi:10.1016/S0040-4020(97)10034-5 - DOI
-
- Baltimore R.S, Christie C.D, Smith G.J. Immunohistopathologic localization of Pseudomonas aeruginosa in lungs from patients with cystic fibrosis. Implications for the pathogenesis of progressive lung deterioration. Am. Rev. Respir. Dis. 1989;140:1650–1661. - PubMed
-
- Bauernfeind A, Bertele R.M, Harms K, Horl G, Jungwirth R, Petermuller C, Przyklenk B, Weisslein-Pfister C. Qualitative and quantitative microbiological analysis of sputa of 102 patients with cystic fibrosis. Infection. 1987;15:270–277. doi:10.1007/BF01644137 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
