Alterations in mitosis and cell cycle progression caused by a mutant lamin A known to accelerate human aging
- PMID: 17360326
- PMCID: PMC1829246
- DOI: 10.1073/pnas.0700854104
Alterations in mitosis and cell cycle progression caused by a mutant lamin A known to accelerate human aging
Abstract
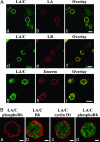
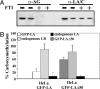
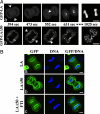
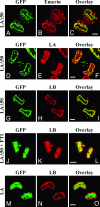
Mutations in the gene encoding nuclear lamin A (LA) cause the premature aging disease Hutchinson-Gilford Progeria Syndrome. The most common of these mutations results in the expression of a mutant LA, with a 50-aa deletion within its C terminus. In this study, we demonstrate that this deletion leads to a stable farnesylation and carboxymethylation of the mutant LA (LADelta50/progerin). These modifications cause an abnormal association of LADelta50/progerin with membranes during mitosis, which delays the onset and progression of cytokinesis. Furthermore, we demonstrate that the targeting of nuclear envelope/lamina components into daughter cell nuclei in early G(1) is impaired in cells expressing LADelta50/progerin. The mutant LA also appears to be responsible for defects in the retinoblastoma protein-mediated transition into S-phase, most likely by inhibiting the hyperphosphorylation of retinoblastoma protein by cyclin D1/cdk4. These results provide insights into the mechanisms responsible for premature aging and also shed light on the role of lamins in the normal process of human aging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Gilford M. Practitioner. 1904;73:188–217.
-
- De-Sandre-Giovannoli A, Bernard R, Cau P, Navarro C, Amiel J, Boccaccio I, Lyonnet S, Stewart CL, Munnich A, Le-Merrer M, Levy N. Science. 2003;300:2055. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

