Ca(2+) sparks operated by membrane depolarization require isoform 3 ryanodine receptor channels in skeletal muscle
- PMID: 17360329
- PMCID: PMC1829292
- DOI: 10.1073/pnas.0700748104
Ca(2+) sparks operated by membrane depolarization require isoform 3 ryanodine receptor channels in skeletal muscle
Erratum in
- Proc Natl Acad Sci U S A. 2007 Aug 14;104(33):13531
Abstract
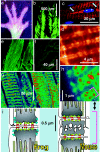
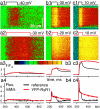
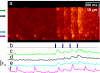
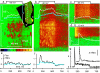
Stimuli are translated to intracellular calcium signals via opening of inositol trisphosphate receptor and ryanodine receptor (RyR) channels of the sarcoplasmic reticulum or endoplasmic reticulum. In cardiac and skeletal muscle of amphibians the stimulus is depolarization of the transverse tubular membrane, transduced by voltage sensors at tubular-sarcoplasmic reticulum junctions, and the unit signal is the Ca(2+) spark, caused by concerted opening of multiple RyR channels. Mammalian muscles instead lose postnatally the ability to produce sparks, and they also lose RyR3, an isoform abundant in spark-producing skeletal muscles. What does it take for cells to respond to membrane depolarization with Ca(2+) sparks? To answer this question we made skeletal muscles of adult mice expressing exogenous RyR3, demonstrated as immunoreactivity at triad junctions. These muscles showed abundant sparks upon depolarization. Sparks produced thusly were found to amplify the response to depolarization in a manner characteristic of Ca(2+)-induced Ca(2+) release processes. The amplification was particularly effective in responses to brief depolarizations, as in action potentials. We also induced expression of exogenous RyR1 or yellow fluorescent protein-tagged RyR1 in muscles of adult mice. In these, tag fluorescence was present at triad junctions. RyR1-transfected muscle lacked voltage-operated sparks. Therefore, the voltage-operated sparks phenotype is specific to the RyR3 isoform. Because RyR3 does not contact voltage sensors, their opening was probably activated by Ca(2+), secondarily to Ca(2+) release through junctional RyR1. Physiologically voltage-controlled Ca(2+) sparks thus require a voltage sensor, a master junctional RyR1 channel that provides trigger Ca(2+), and a slave parajunctional RyR3 cohort.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
RyR1-specific requirement for depolarization-induced Ca2+ sparks in urinary bladder smooth muscle.J Cell Sci. 2007 Nov 1;120(Pt 21):3784-91. doi: 10.1242/jcs.009415. Epub 2007 Oct 9. J Cell Sci. 2007. PMID: 17925380
-
Imperatoxin a enhances Ca(2+) release in developing skeletal muscle containing ryanodine receptor type 3.Biophys J. 2002 Mar;82(3):1319-28. doi: 10.1016/S0006-3495(02)75487-8. Biophys J. 2002. PMID: 11867448 Free PMC article.
-
RYR1 and RYR3 have different roles in the assembly of calcium release units of skeletal muscle.Biophys J. 2000 Nov;79(5):2494-508. doi: 10.1016/S0006-3495(00)76491-5. Biophys J. 2000. PMID: 11053125 Free PMC article.
-
Putative roles of type 3 ryanodine receptor isoforms (RyR3).Trends Cardiovasc Med. 2000 Feb;10(2):65-70. doi: 10.1016/s1050-1738(00)00050-5. Trends Cardiovasc Med. 2000. PMID: 11150732 Review.
-
Two ryanodine receptor isoforms in nonmammalian vertebrate skeletal muscle: possible roles in excitation-contraction coupling and other processes.Prog Biophys Mol Biol. 2011 May;105(3):134-44. doi: 10.1016/j.pbiomolbio.2010.10.003. Epub 2010 Oct 26. Prog Biophys Mol Biol. 2011. PMID: 21029746 Review.
Cited by
-
Role of ryanodine receptor subtypes in initiation and formation of calcium sparks in arterial smooth muscle: comparison with striated muscle.J Biomed Biotechnol. 2009;2009:135249. doi: 10.1155/2009/135249. Epub 2009 Dec 8. J Biomed Biotechnol. 2009. PMID: 20029633 Free PMC article. Review.
-
Altered expression of triadin 95 causes parallel changes in localized Ca2+ release events and global Ca2+ signals in skeletal muscle cells in culture.J Physiol. 2008 Dec 1;586(23):5803-18. doi: 10.1113/jphysiol.2008.160457. Epub 2008 Oct 9. J Physiol. 2008. PMID: 18845610 Free PMC article.
-
Calsequestrin depolymerizes when calcium is depleted in the sarcoplasmic reticulum of working muscle.Proc Natl Acad Sci U S A. 2017 Jan 24;114(4):E638-E647. doi: 10.1073/pnas.1620265114. Epub 2017 Jan 9. Proc Natl Acad Sci U S A. 2017. PMID: 28069951 Free PMC article.
-
Mitochondrial calcium uptake regulates rapid calcium transients in skeletal muscle during excitation-contraction (E-C) coupling.J Biol Chem. 2011 Sep 16;286(37):32436-43. doi: 10.1074/jbc.M110.217711. Epub 2011 Jul 27. J Biol Chem. 2011. PMID: 21795684 Free PMC article.
-
High Time Resolution Analysis of Voltage-Dependent and Voltage-Independent Calcium Sparks in Frog Skeletal Muscle Fibers.Front Physiol. 2020 Dec 15;11:599822. doi: 10.3389/fphys.2020.599822. eCollection 2020. Front Physiol. 2020. PMID: 33384612 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

