Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand
- PMID: 17360345
- PMCID: PMC1829280
- DOI: 10.1073/pnas.0700293104
Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand
Abstract
We evolved muscarinic receptors in yeast to generate a family of G protein-coupled receptors (GPCRs) that are activated solely by a pharmacologically inert drug-like and bioavailable compound (clozapine-N-oxide). Subsequent screening in human cell lines facilitated the creation of a family of muscarinic acetylcholine GPCRs suitable for in vitro and in situ studies. We subsequently created lines of telomerase-immortalized human pulmonary artery smooth muscle cells stably expressing all five family members and found that each one faithfully recapitulated the signaling phenotype of the parent receptor. We also expressed a G(i)-coupled designer receptor in hippocampal neurons (hM(4)D) and demonstrated its ability to induce membrane hyperpolarization and neuronal silencing. We have thus devised a facile approach for designing families of GPCRs with engineered ligand specificities. Such reverse-engineered GPCRs will prove to be powerful tools for selectively modulating signal-transduction pathways in vitro and in vivo.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


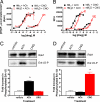

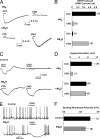
Comment in
-
New tools to build synthetic hormonal pathways.Proc Natl Acad Sci U S A. 2007 Mar 20;104(12):4777-8. doi: 10.1073/pnas.0700913104. Epub 2007 Mar 12. Proc Natl Acad Sci U S A. 2007. PMID: 17360323 Free PMC article. No abstract available.
References
-
- Armbruster BN, Roth BL. J Biol Chem. 2004;280:5129–5132. - PubMed
-
- Bishop A, Buzko O, Heyeck-Dumas S, Jung I, Kraybill B, Liu Y, Shah K, Ulrich S, Witucki L, Yang F, et al. Annu Rev Biophys Biomol Struct. 2000;29:577–606. - PubMed
-
- Scearce-Levie K, Coward P, Redfern CH, Conklin BR. Trends Pharmacol Sci. 2001;22:414–420. - PubMed
-
- Redfern CH, Coward P, Degtyarev MY, Lee EK, Kwa AT, Hennighausen L, Bujard H, Fishman GI, Conklin BR. Nat Biotechnol. 1999;17:165–169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

