Cytoskeletal dynamics of human erythrocyte
- PMID: 17360346
- PMCID: PMC1829243
- DOI: 10.1073/pnas.0700257104
Cytoskeletal dynamics of human erythrocyte
Abstract
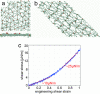
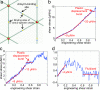
The human erythrocyte (red blood cell, RBC) demonstrates extraordinary ability to undergo reversible large deformation and fluidity. Such mechanical response cannot be consistently rationalized on the basis of fixed connectivity of the cell cytoskeleton that comprises the spectrin molecular network tethered to phospholipid membrane. Active topological remodeling of spectrin network has been postulated, although detailed models of such dynamic reorganization are presently unavailable. Here we present a coarse-grained cytoskeletal dynamics simulation with breakable protein associations to elucidate the roles of shear stress, specific chemical agents, and thermal fluctuations in cytoskeleton remodeling. We demonstrate a clear solid-to-fluid transition depending on the metabolic energy influx. The solid network's plastic deformation also manifests creep and yield regimes depending on the strain rate. This cytoskeletal dynamics model offers a means to resolve long-standing questions regarding the reference state used in RBC elasticity theory for determining the equilibrium shape and deformation response. In addition, the simulations offer mechanistic insights into the onset of plasticity and void percolation in cytoskeleton. These phenomena may have implication for RBC membrane loss and shape change in the context of hereditary hemolytic disorders such as spherocytosis and elliptocytosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Bennett V. Annu Rev Biochem. 1985;54:273–304. - PubMed
-
- Mohandas N, Evans E. Annu Rev Biophys Biomol Struct. 1994;23:787–818. - PubMed
-
- An XL, Lecomte MC, Chasis JA, Mohandas N, Gratzer W. J Biol Chem. 2002;277:31796–31800. - PubMed
-
- Cloitre M, Borrega R, Leibler L. Phys Rev Lett. 2000;85:4819–4822. - PubMed
-
- Bursac P, Lenormand G, Fabry B, Oliver M, Weitz DA, Viasnoff V, Butler JP, Fredberg JJ. Nat Mater. 2005;4:557–561. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

