Sensitivity of T cells to antigen and antagonism emerges from differential regulation of the same molecular signaling module
- PMID: 17360359
- PMCID: PMC1838481
- DOI: 10.1073/pnas.0611482104
Sensitivity of T cells to antigen and antagonism emerges from differential regulation of the same molecular signaling module
Abstract
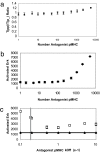
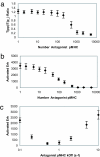
Activation of T helper cells is necessary for the adaptive immune response to pathogens, and spurious activation can result in organ-specific autoimmunity (e.g., multiple sclerosis). T cell activation is initiated by membrane-proximal signaling that is predicated on the binding of the T cell receptor expressed on the T cell surface to peptide major histocompatibility complex (pMHC) molecules presented on the surface of antigen-presenting cells. These signaling processes regulate diverse outcomes, such as the ability of T cells to discriminate sensitively between stimulatory pMHC molecules and those that are characteristic of "self," and the phenomenon of antagonism (wherein the presence of certain pMHC molecules impairs T cell receptor signaling). We describe a molecular model for membrane-proximal signaling in T cells from which these disparate observations emerge as two sides of the same coin. This development of a unified mechanism that is consistent with diverse data would not have been possible without explicit consideration of the stochastic nature of the pertinent biochemical events. Our studies also reveal that certain previously proposed concepts are not dueling ideas but rather are different stimuli-dependent manifestations of a unified molecular model for membrane-proximal signaling. This model may provide a conceptual framework for further investigations of early events that regulate T cell activation in response to self and foreign antigens and for the development of intervention protocols to inhibit aberrant signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM. Nature. 2002;419:845–849. - PubMed
-
- Purbhoo MA, Irvine DJ, Huppa JB, Davis MM. Nat Immunol. 2004;5:524–530. - PubMed
-
- Krogsgaard M, Li QJ, Sumen C, Huppa JB, Huse M, Davis MM. Nature. 2005;434:238–243. - PubMed
-
- Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. Science. 1999;285:221–227. - PubMed
-
- Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Nature. 1998;395:82–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

