On the thermodynamic stability of a charged arginine side chain in a transmembrane helix
- PMID: 17360368
- PMCID: PMC1829244
- DOI: 10.1073/pnas.0610470104
On the thermodynamic stability of a charged arginine side chain in a transmembrane helix
Abstract
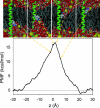
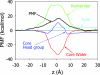
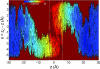
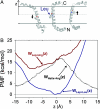
Biological membranes consist of bilayer arrangements of lipids forming a hydrophobic core that presents a physical barrier to all polar and charged molecules. This long-held notion has recently been challenged by biological translocon-based experiments that report small apparent free energies to insert charged side chains near the center of a transmembrane (TM) helix. We have carried out fully atomistic simulations to provide the free-energy profile for moving a TM helix containing a protonated Arg side chain across a lipid bilayer. Our results reveal the fundamental thermodynamics governing the stability of charged side chains in membranes and the microscopic interactions involved. Despite local membrane deformations, where large amounts of water and lipid head groups are pulled into the bilayer to interact with Arg, the free-energy barrier is 17 kcal/mol. We provide a rationale for the differences in our microscopic free energies and cell biological experiments using free-energy calculations that indicate that a protonated Arg at the central residue of a TM helix of the Leader peptidase might reside close to the interface and not at the membrane center. Our findings have implications for the gating mechanisms of voltage-gated ion channels, suggesting that movements of protonated Arg residues through the membrane will be prohibited.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Gennis R. Biomembranes: Molecular Structure and Functions. New York: Springer; 1989.
-
- Yang A-S, Honig B. Curr Opin Struct Biol. 1992;2:40–45.
-
- Huang HC, Briggs JM. Biopolymers. 2002;63:247–260. - PubMed
-
- Kalderon D, Richardson WD, Markham AF, Smith AE. Nature. 1984;311:33–38. - PubMed
-
- Han X, Bushweller JH, Cafiso DS, Tamm LK. Nat Struct Biol. 2001;8:715–720. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

