S-adenosylmethionine directly inhibits binding of 30S ribosomal subunits to the SMK box translational riboswitch RNA
- PMID: 17360376
- PMCID: PMC1829232
- DOI: 10.1073/pnas.0609956104
S-adenosylmethionine directly inhibits binding of 30S ribosomal subunits to the SMK box translational riboswitch RNA
Abstract
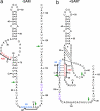
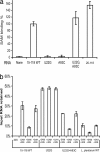
The S(MK) box is a conserved riboswitch motif found in the 5' untranslated region of metK genes [encoding S-adenosylmethionine (SAM) synthetase] in lactic acid bacteria, including Enterococcus, Streptococcus, and Lactococcus sp. Previous studies showed that this RNA element binds SAM in vitro, and SAM binding causes a structural rearrangement that sequesters the Shine-Dalgarno (SD) sequence by pairing with an anti-SD (ASD) element. A model was proposed in which SAM binding inhibits metK translation by preventing binding of the ribosome to the SD region of the mRNA. In the current work, the addition of SAM was shown to inhibit binding of 30S ribosomal subunits to S(MK) box RNA; in contrast, the addition of S-adenosylhomocysteine (SAH) had no effect. A mutant RNA, which has a disrupted SD-ASD pairing, was defective in SAM binding and showed no reduction of ribosome binding in the presence of SAM, whereas a compensatory mutation that restored SD-ASD pairing restored the response to SAM. Primer extension inhibition assays provided further evidence for SD-ASD pairing in the presence of SAM. These results strongly support the model that S(MK) box translational repression operates through occlusion of the ribosome binding site and that SAM binding requires the SD-ASD pairing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Grundy FJ, Henkin TM. Curr Opin Microbiol. 2004;7:126–131. - PubMed
-
- Vitreschak AG, Rodionov DA, Mironov AA, Gelfand MS. Trends Genet. 2004;20:44–50. - PubMed
-
- Nudler E, Mironov AS. Trends Biochem. 2004;29:11–17. - PubMed
-
- Winkler WC, Breaker RR. Annu Rev Microbiol. 2005;59:487–517. - PubMed
-
- Grundy FJ, Henkin TM. Crit Rev Biochem Mol Biol. 2006;41:329–338. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

