Genetic deletion of p66(Shc) adaptor protein prevents hyperglycemia-induced endothelial dysfunction and oxidative stress
- PMID: 17360381
- PMCID: PMC1829289
- DOI: 10.1073/pnas.0609656104
Genetic deletion of p66(Shc) adaptor protein prevents hyperglycemia-induced endothelial dysfunction and oxidative stress
Abstract
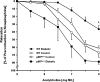
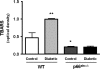
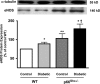
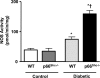
Increased production of reactive oxygen species (ROS) and loss of endothelial NO bioavailability are key features of vascular disease in diabetes mellitus. The p66(Shc) adaptor protein controls cellular responses to oxidative stress. Mice lacking p66(Shc) (p66(Shc-/-)) have increased resistance to ROS and prolonged life span. The present work was designed to investigate hyperglycemia-associated changes in endothelial function in a model of insulin-dependent diabetes mellitus p66(Shc-/-) mouse. p66(Shc-/-) and wild-type (WT) mice were injected with citrate buffer (control) or made diabetic by an i.p. injection of 200 mg of streptozotocin per kg of body weight. Streptozotocin-treated p66(Shc-/-) and WT mice showed a similar increase in blood glucose. However, significant differences arose with respect to endothelial dysfunction and oxidative stress. WT diabetic mice displayed marked impairment of endothelium-dependent relaxations, increased peroxynitrite (ONOO(-)) generation, nitrotyrosine expression, and lipid peroxidation as measured in the aortic tissue. In contrast, p66(Shc-/-) diabetic mice did not develop these high-glucose-mediated abnormalities. Furthermore, protein expression of the antioxidant enzyme heme oxygenase 1 and endothelial NO synthase were up-regulated in p66(Shc-/-) but not in WT mice. We report that p66(Shc-/-) mice are resistant to hyperglycemia-induced, ROS-dependent endothelial dysfunction. These data suggest that p66(Shc) adaptor protein is part of a signal transduction pathway relevant to hyperglycemia vascular damage and, hence, may represent a novel therapeutic target against diabetic vascular complications.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Creager MA, Luscher TF, Cosentino F, Beckman JA. Circulation. 2003;108:1527–1532. - PubMed
-
- Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. J Am Coll Cardiol. 2003;42:1149–1160. - PubMed
-
- Lerman A, Zeiher AM. Circulation. 2005;111:363–368. - PubMed
-
- Turko IV, Murad F. Pharmacol Rev. 2002;54:619–634. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

