Change in permeant size selectivity by phosphorylation of connexin 43 gap-junctional hemichannels by PKC
- PMID: 17360407
- PMCID: PMC1817834
- DOI: 10.1073/pnas.0603154104
Change in permeant size selectivity by phosphorylation of connexin 43 gap-junctional hemichannels by PKC
Abstract
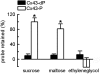
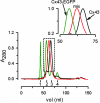
Gap-junctional channels, permeable to large hydrophilic solutes of up to M(r) approximately 1,000, are responsible for cell-to-cell communication. Phosphorylation of connexin 43 (Cx43) by PKC abolishes the permeability of gap-junctional channels and hemichannels to large hydrophilic solutes, but not to small inorganic ions. Here, we report on a methodology to produce purified hemichannels of controlled subunit composition and apply it to the generation of hemichannels with variable number of PKC-phosphorylated subunits. The subunit composition was determined by luminescence resonance energy transfer. We show that all Cx43 subunits in the hemichannel hexamer have to be phosphorylated to abolish sucrose (M(r) 342) permeability. We also show that the hemichannel pores with all subunits phosphorylated by PKC have a sizable diameter, allowing for permeation of the small hydrophilic solute ethyleneglycol (M(r) 62). These results indicate that phosphorylation of Cx43 by PKC alters the hemichannel size selectivity and explain why PKC activity affects dye transfer between cells without consistent effects on electrical communication.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
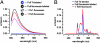


References
-
- Harris AL. Q Rev Biophys. 2001;34:325–472. - PubMed
-
- Sosinsky GE, Nicholson BJ. Biochim Biophys Acta. 2005;1711:99–125. - PubMed
-
- Unger VM, Kumar NM, Gilula NBM, Yeager M. Nat Struct Biol. 1997;4:39–43. - PubMed
-
- Goldberg GS, Valiunas V, Brink PR. Biochim Biophys Acta. 2004;1662:96–101. - PubMed
-
- Dhein S. Cardiovasc Res. 2004;62:287–298. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

