Adhesion mechanisms of the mussel foot proteins mfp-1 and mfp-3
- PMID: 17360430
- PMCID: PMC1820661
- DOI: 10.1073/pnas.0607852104
Adhesion mechanisms of the mussel foot proteins mfp-1 and mfp-3
Abstract
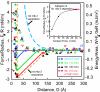
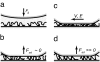
Mussels adhere to a variety of surfaces by depositing a highly specific ensemble of 3,4-dihydroxyphenyl-l-alanine (DOPA) containing proteins. The adhesive properties of Mytilus edulis foot proteins mfp-1 and mfp-3 were directly measured at the nano-scale by using a surface forces apparatus (SFA). An adhesion energy of order W approximately 3 x 10(-4) J/m(2) was achieved when separating two smooth and chemically inert surfaces of mica (a common alumino-silicate clay mineral) bridged or "glued" by mfp-3. This energy corresponds to an approximate force per plaque of approximately 100 gm, more than enough to hold a mussel in place if no peeling occurs. In contrast, no adhesion was detected between mica surfaces bridged by mfp-1. AFM imaging and SFA experiments showed that mfp-1 can adhere well to one mica surface, but is unable to then link to another (unless sheared), even after prolonged contact time or increased load (pressure). Although mechanistic explanations for the different behaviors are not yet possible, the results are consistent with the apparent function of the proteins, i.e., mfp-1 is disposed as a "protective" coating, and mfp-3 as the adhesive or "glue" that binds mussels to surfaces. The results suggest that the adhesion on mica is due to weak physical interactions rather than chemical bonding, and that the strong adhesion forces of plaques arise as a consequence of their geometry (e.g., their inability to be peeled off) rather than a high intrinsic surface or adhesion energy, W.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Bridging adhesion of mussel-inspired peptides: role of charge, chain length, and surface type.Langmuir. 2015 Jan 27;31(3):1105-12. doi: 10.1021/la504316q. Epub 2015 Jan 12. Langmuir. 2015. PMID: 25540823 Free PMC article.
-
Mussel adhesive protein provides cohesive matrix for collagen type-1α.Biomaterials. 2015 May;51:51-57. doi: 10.1016/j.biomaterials.2015.01.033. Epub 2015 Feb 17. Biomaterials. 2015. PMID: 25770997 Free PMC article.
-
Adhesion of mussel foot proteins to different substrate surfaces.J R Soc Interface. 2013 Feb;10(79):20120759. doi: 10.1098/rsif.2012.0759. J R Soc Interface. 2013. PMID: 23173195 Free PMC article.
-
Mussel-designed protective coatings for compliant substrates.J Dent Res. 2008 Aug;87(8):701-9. doi: 10.1177/154405910808700808. J Dent Res. 2008. PMID: 18650539 Free PMC article. Review.
-
Mussel adhesion - essential footwork.J Exp Biol. 2017 Feb 15;220(Pt 4):517-530. doi: 10.1242/jeb.134056. J Exp Biol. 2017. PMID: 28202646 Free PMC article. Review.
Cited by
-
Injectable citrate-based mussel-inspired tissue bioadhesives with high wet strength for sutureless wound closure.Biomaterials. 2012 Nov;33(32):7972-83. doi: 10.1016/j.biomaterials.2012.07.055. Epub 2012 Aug 16. Biomaterials. 2012. PMID: 22902057 Free PMC article.
-
Sustainable polycarbonate adhesives for dry and aqueous conditions with thermoresponsive properties.Nat Commun. 2019 Dec 2;10(1):5478. doi: 10.1038/s41467-019-13449-y. Nat Commun. 2019. PMID: 31792214 Free PMC article.
-
Direct Observation of the Interplay of Catechol Binding and Polymer Hydrophobicity in a Mussel-Inspired Elastomeric Adhesive.ACS Cent Sci. 2018 Oct 24;4(10):1420-1429. doi: 10.1021/acscentsci.8b00526. Epub 2018 Oct 9. ACS Cent Sci. 2018. PMID: 30410980 Free PMC article.
-
Climbing plants: attachment adaptations and bioinspired innovations.Plant Cell Rep. 2018 Apr;37(4):565-574. doi: 10.1007/s00299-017-2240-y. Epub 2017 Nov 29. Plant Cell Rep. 2018. PMID: 29188422 Review.
-
Peptide Length and Dopa Determine Iron-Mediated Cohesion of Mussel Foot Proteins.Adv Funct Mater. 2015 Sep 23;25(36):5840-5847. doi: 10.1002/adfm.201502256. Epub 2015 Aug 17. Adv Funct Mater. 2015. PMID: 28670243 Free PMC article.
References
-
- Waite JH, Andersen NH, Jewhurst S, Sun CJ. J Adhes. 2005;81:297–317.
-
- Waite JH, Qin XX, Coyne KJ. Matrix Biol. 1998;17:93–106. - PubMed
-
- Benedict CV, Waite JH. J Morphol. 1986;189:171–181. - PubMed
-
- Sun CJ, Waite JH. J Biol Chem. 2005;280:39332–39336. - PubMed
-
- Deacon MP, Davis SS, Waite JH, Harding SE. Biochemistry. 1998;37:14108–14112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

