Cross-talk between Chk1 and Chk2 in double-mutant thymocytes
- PMID: 17360434
- PMCID: PMC1820665
- DOI: 10.1073/pnas.0611584104
Cross-talk between Chk1 and Chk2 in double-mutant thymocytes
Erratum in
- Proc Natl Acad Sci U S A. 2007 May 8;104(19):8196. Liu, Qinghua [added]
-
Correction for Zaugg et al., Cross-talk between Chk1 and Chk2 in double-mutant thymocytes.Proc Natl Acad Sci U S A. 2023 Dec 19;120(51):e2320119120. doi: 10.1073/pnas.2320119120. Epub 2023 Dec 15. Proc Natl Acad Sci U S A. 2023. PMID: 38100421 Free PMC article. No abstract available.
Abstract
Chk1 is a checkpoint kinase and an important regulator of mammalian cell division. Because null mutation of Chk1 in mice is embryonic lethal, we used the Cre-loxP system and the Lck promoter to generate conditional mutant mice in which Chk1 was deleted only in the T lineage. In the absence of Chk1, the transition of CD4(-)CD8(-) double-negative (DN) thymocytes to CD4(+)CD8(+) double-positive (DP) cells was blocked due to an increase in apoptosis at the DN2 and DN3 stages. Strikingly, loss of Chk1 activated the checkpoint kinase Chk2 as well as the tumor suppressor p53 in these thymocytes. However, the developmental defects caused by Chk1 deletion were not rescued by p53 inactivation. Significantly, even though Chk1 deletion is highly lethal in proliferating tissues, we succeeded in using in vivo methods to generate Chk1/Chk2 double-knockout T cells. Analysis of these T cells revealed an interesting interaction between Chk1 and Chk2 functions that partially rescued the apoptosis of the double-mutant cells. Thus, Chk1 is both critical for the survival of proliferating cells and engages in cross-talk with the Chk2 checkpoint kinase pathway. These factors have implications for the targeting of Chk1 as an anticancer therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


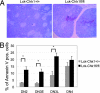


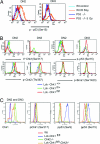
Similar articles
-
Chk2 is a tumor suppressor that regulates apoptosis in both an ataxia telangiectasia mutated (ATM)-dependent and an ATM-independent manner.Mol Cell Biol. 2002 Sep;22(18):6521-32. doi: 10.1128/MCB.22.18.6521-6532.2002. Mol Cell Biol. 2002. PMID: 12192050 Free PMC article.
-
Cooperative functions of Chk1 and Chk2 reduce tumour susceptibility in vivo.EMBO J. 2010 Oct 20;29(20):3558-70. doi: 10.1038/emboj.2010.218. Epub 2010 Sep 10. EMBO J. 2010. PMID: 20834228 Free PMC article.
-
Knockdown of Chk1 sensitizes human colon carcinoma HCT116 cells in a p53-dependent manner to lidamycin through abrogation of a G2/M checkpoint and induction of apoptosis.Cancer Biol Ther. 2009 Aug;8(16):1559-66. doi: 10.4161/cbt.8.16.8955. Epub 2009 Aug 8. Cancer Biol Ther. 2009. PMID: 19502782
-
Chk1 and Chk2 kinases in checkpoint control and cancer.Cancer Cell. 2003 May;3(5):421-9. doi: 10.1016/s1535-6108(03)00110-7. Cancer Cell. 2003. PMID: 12781359 Review.
-
The multiple checkpoint functions of CHK1 and CHK2 in maintenance of genome stability.Front Biosci. 2008 May 1;13:5016-29. doi: 10.2741/3060. Front Biosci. 2008. PMID: 18508566 Review.
Cited by
-
Checkpoint kinase1 (CHK1) is an important biomarker in breast cancer having a role in chemotherapy response.Br J Cancer. 2015 Mar 3;112(5):901-11. doi: 10.1038/bjc.2014.576. Epub 2015 Feb 17. Br J Cancer. 2015. PMID: 25688741 Free PMC article.
-
Roles of Chk1 in cell biology and cancer therapy.Int J Cancer. 2014 Mar 1;134(5):1013-23. doi: 10.1002/ijc.28226. Epub 2013 May 28. Int J Cancer. 2014. PMID: 23613359 Free PMC article. Review.
-
The DNA damage effector Chk1 kinase regulates Cdc14B nucleolar shuttling during cell cycle progression.Cell Cycle. 2011 Feb 15;10(4):671-9. doi: 10.4161/cc.10.4.14901. Epub 2011 Feb 15. Cell Cycle. 2011. PMID: 21301228 Free PMC article.
-
Checkpoint kinase 1 is essential for normal B cell development and lymphomagenesis.Nat Commun. 2017 Nov 22;8(1):1697. doi: 10.1038/s41467-017-01850-4. Nat Commun. 2017. PMID: 29167438 Free PMC article.
-
Oxaliplatin responses in colorectal cancer cells are modulated by CHK2 kinase inhibitors.Br J Pharmacol. 2010 Mar;159(6):1326-38. doi: 10.1111/j.1476-5381.2009.00607.x. Epub 2010 Jan 28. Br J Pharmacol. 2010. PMID: 20128802 Free PMC article.
References
-
- Harris SL, Levine AJ. Oncogene. 2005;24:2899–2908. - PubMed
-
- Nevanlinna H, Bartek J. Oncogene. 2006;25:5912–5919. - PubMed
-
- Shiloh Y. Natl Rev Cancer. 2003;3:155–168. - PubMed
-
- Zhou BB, Elledge SJ. Nature. 2000;408:433–439. - PubMed
-
- Gatei M, Sloper K, Sorensen C, Syljuasen R, Falck J, Hobson K, Savage K, Lukas J, Zhou BB, Bartek J, Khanna KK. J Biol Chem. 2003;278:14806–14811. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

