E-Syts, a family of membranous Ca2+-sensor proteins with multiple C2 domains
- PMID: 17360437
- PMCID: PMC1820668
- DOI: 10.1073/pnas.0611725104
E-Syts, a family of membranous Ca2+-sensor proteins with multiple C2 domains
Abstract
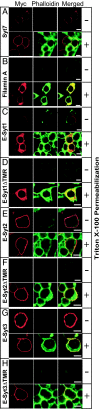
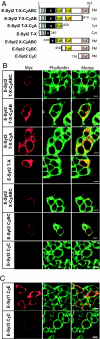
C(2) domains are autonomously folded protein modules that generally act as Ca(2+)- and phospholipid-binding domains and/or as protein-protein interaction domains. We now report the primary structures and biochemical properties of a family of evolutionarily conserved mammalian proteins, referred to as E-Syts, for extended synaptotagmin-like proteins. E-Syts contain an N-terminal transmembrane region, a central juxtamembranous domain that is conserved from yeast to human, and five (E-Syt1) or three (E-Syt2 and E-Syt3) C-terminal C(2) domains. Only the first E-Syt C(2) domain, the C(2)A domain, includes the complete sequence motif that is required for Ca(2+) binding in C(2) domains. Recombinant protein fragments of E-Syt2 that include the first C(2) domain are capable of Ca(2+)-dependent phospholipid binding at micromolar concentrations of free Ca(2+), suggesting that E-Syts bind Ca(2+) through their first C(2) domain in a phospholipid complex. E-Syts are ubiquitously expressed, but enriched in brain. Expression of myc-tagged E-Syt proteins in transfected cells demonstrated localization to intracellular membranes for E-Syt1 and to plasma membranes for E-Syt2 and E-Syt3. Structure/function studies showed that the plasma-membrane localization of E-Syt2 and E-Syt3 was directed by their C-terminal C(2)C domains. This result reveals an unexpected mechanism by which the C(2)C domains of E-Syt2 and E-Syt3 functions as a targeting motif that localizes these proteins into the plasma membrane independent of their transmembrane region. Viewed together, our findings suggest that E-Syts function as Ca(2+)-regulated intrinsic membrane proteins with multiple C(2) domains, expanding the repertoire of such proteins to a fourth class beyond synaptotagmins, ferlins, and MCTPs (multiple C(2) domain and transmembrane region proteins).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Ca(2+)-dependent and -independent activities of neural and non-neural synaptotagmins.Nature. 1995 Jun 15;375(6532):594-9. doi: 10.1038/375594a0. Nature. 1995. PMID: 7791877
-
The C2A domain of synaptotagmin-like protein 3 (Slp3) is an atypical calcium-dependent phospholipid-binding machine: comparison with the C2A domain of synaptotagmin I.Biochem J. 2002 Sep 1;366(Pt 2):681-7. doi: 10.1042/BJ20020484. Biochem J. 2002. PMID: 12049610 Free PMC article.
-
Structural characterization of soluble E-Syt2.FEBS Lett. 2008 Nov 26;582(28):3941-7. doi: 10.1016/j.febslet.2008.10.038. Epub 2008 Oct 31. FEBS Lett. 2008. PMID: 18977228
-
The mast cell: where endocytosis and regulated exocytosis meet.Immunol Rev. 2007 Jun;217:292-303. doi: 10.1111/j.1600-065X.2007.00516.x. Immunol Rev. 2007. PMID: 17498067 Review.
-
Endoplasmic Reticulum - Plasma Membrane Crosstalk Mediated by the Extended Synaptotagmins.Adv Exp Med Biol. 2017;997:83-93. doi: 10.1007/978-981-10-4567-7_6. Adv Exp Med Biol. 2017. PMID: 28815523 Review.
Cited by
-
ER-PM connections: sites of information transfer and inter-organelle communication.Curr Opin Cell Biol. 2013 Aug;25(4):434-42. doi: 10.1016/j.ceb.2013.02.020. Epub 2013 Mar 20. Curr Opin Cell Biol. 2013. PMID: 23522446 Free PMC article. Review.
-
Extended-synaptotagmin 1 engages in unconventional protein secretion mediated via SEC22B+ vesicle pathway in liver cancer.Proc Natl Acad Sci U S A. 2022 Sep 6;119(36):e2202730119. doi: 10.1073/pnas.2202730119. Epub 2022 Aug 31. Proc Natl Acad Sci U S A. 2022. PMID: 36044553 Free PMC article.
-
The role of extended synaptotagmin at membrane contact sites in cancer research.Front Cell Dev Biol. 2023 Nov 27;11:1291506. doi: 10.3389/fcell.2023.1291506. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38089882 Free PMC article. Review.
-
Hypothalamic extended synaptotagmin-3 contributes to the development of dietary obesity and related metabolic disorders.Proc Natl Acad Sci U S A. 2020 Aug 18;117(33):20149-20158. doi: 10.1073/pnas.2004392117. Epub 2020 Aug 3. Proc Natl Acad Sci U S A. 2020. PMID: 32747560 Free PMC article.
-
Tricalbin-Mediated Contact Sites Control ER Curvature to Maintain Plasma Membrane Integrity.Dev Cell. 2019 Nov 18;51(4):476-487.e7. doi: 10.1016/j.devcel.2019.10.018. Dev Cell. 2019. PMID: 31743662 Free PMC article.
References
-
- Coussens L, Parker PJ, Rhee L, Yang-Feng TL, Chen E, Waterfield MD, Francke U, Ullrich A. Science. 1986;233:859–866. - PubMed
-
- Davletov BA, Südhof TC. J Biol Chem. 1993;268:26386–26390. - PubMed
-
- Chapman ER, Jahn R. J Biol Chem. 1994;269:5735–5741. - PubMed
-
- Persechini A, Moncrief ND, Kretsinger RH. Trends Neurosci. 1989;12:462–467. - PubMed
-
- Sutton RB, Davletov BA, Berghuis AM, Südhof TC, Sprang SR. Cell. 1995;80:929–938. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

