KCNQ potassium channel mutations cause cardiac arrhythmias in Drosophila that mimic the effects of aging
- PMID: 17360457
- PMCID: PMC1820688
- DOI: 10.1073/pnas.0609278104
KCNQ potassium channel mutations cause cardiac arrhythmias in Drosophila that mimic the effects of aging
Abstract
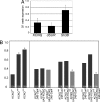
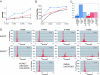
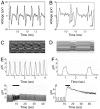
Population profiles of industrialized countries show dramatic increases in cardiovascular disease with age, but the molecular and genetic basis of disease progression has been difficult to study because of the lack of suitable model systems. Our studies of Drosophila show a markedly elevated incidence of cardiac dysfunction and arrhythmias in aging fruit fly hearts and a concomitant decrease in the expression of the Drosophila homolog of human KCNQ1-encoded K(+) channel alpha subunits. In humans, this channel is involved in myocardial repolarization, and alterations in the function of this channel are associated with an increased risk for Torsades des Pointes arrhythmias and sudden death. Hearts from young KCNQ1 mutant fruit flies exhibit prolonged contractions and fibrillations reminiscent of Torsades des Pointes arrhythmias, and they exhibit severely increased susceptibility to pacing-induced cardiac dysfunction at young ages, characteristics that are observed only at advanced ages in WT flies. The fibrillations observed in mutant flies correlate with delayed relaxation of the myocardium, as revealed by increases in the duration of phasic contractions, extracellular field potentials, and in the baseline diastolic tension. These results suggest that K(+) currents, mediated by a KCNQ channel, contribute to the repolarization reserve of fly hearts, ensuring normal excitation-contraction coupling and rhythmical contraction. That arrhythmias in both WT and KCNQ1 mutants become worse as flies age suggests that additional factors are also involved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Thom TNH, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng Z, Flegal K, O'Donnell C, Kittner S, Lloyd-Jones D, et al. Circulation. 2006;113:e85–e151. - PubMed
-
- Lakatta EG. Heart Fail Rev. 2002;7:29–49. - PubMed
-
- Lakatta EG, Levy D. Circulation. 2003;107:346–354. - PubMed
-
- Priori SG, Napolitano C. Ann NY Acad Sci. 2004;1015:96–110. - PubMed
-
- Bodmer R. Trends Cardiovasc Med. 1995;5:21–27. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

