Invariant NKT cells sustain specific B cell responses and memory
- PMID: 17360464
- PMCID: PMC1805488
- DOI: 10.1073/pnas.0700191104
Invariant NKT cells sustain specific B cell responses and memory
Abstract
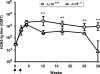
Invariant natural killer T (iNKT) cells are innate-like lymphocytes recognizing CD1d-restricted glycolipid antigens, such as alpha-galactosylceramide (alphaGC). We assessed whether iNKT cells help B lymphocyte responses and found that mice immunized with proteins and alphaGC develop antibody titers 1-2 logs higher than those induced by proteins alone. Activation of iNKT cells enhances protection against infections such as influenza and elicits higher frequencies of memory B cells and higher antibody responses to booster immunizations. Protein vaccination with alphaGC, but not with conventional adjuvants, elicits IgG responses in mice lacking MHC class II molecules, demonstrating that iNKT cells can substitute for CD4(+) T cell help to B cells. Interestingly, the decay of circulating antibodies is faster in mice lacking iNKT cells. These findings point to a homeostatic role for iNKT cells on critical features of the antibody response such as immunity and B cell memory.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

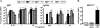
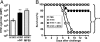
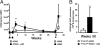

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

