Immunotherapeutic activity of a conjugate of a Toll-like receptor 7 ligand
- PMID: 17360465
- PMCID: PMC1820696
- DOI: 10.1073/pnas.0611624104
Immunotherapeutic activity of a conjugate of a Toll-like receptor 7 ligand
Abstract
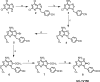
The immunotherapeutic activity of Toll-like receptor (TLR) activators has been difficult to exploit because of side effects related to the release and systemic dispersion of proinflammatory cytokines. To overcome this barrier, we have synthesized a versatile TLR7 agonist, 4-[6-amino-8-hydroxy-2-(2-methoxyethoxy)purin-9-ylmethyl]benzaldehyde (UC-1V150), bearing a free aldehyde that could be coupled to many different auxiliary chemical entities through a linker molecule with a hydrazine or amino group without any loss of activity. UC-1V150 was covalently coupled to mouse serum albumin (MSA) at a 5:1 molar ratio to yield a stable molecule with a characteristically altered UV spectrum. Compared with the unconjugated TLR7 agonist, the UC-1V150/MSA was a 10- to 100-fold more potent inducer of cytokine production in vitro by mouse bone marrow-derived macrophage and human peripheral blood mononuclear cells. When administrated to the lung, the conjugate induced a prolonged local release of cytokines at levels 10-fold or more higher than those found in serum. Under the same conditions, the untethered TLR7 ligand induced quick systemic cytokine release with resultant toxicity. In addition, two pulmonary infectious disease models were investigated wherein mice were pretreated with the conjugate and then challenged with either Bacillus anthracis spores or H1N1 influenza A virus. Significant delay in mortality was observed in both disease models with UC-1V150/MSA-pretreated mice, indicating the potential usefulness of the conjugate as a localized and targeted immunotherapeutic agent.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


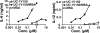


Similar articles
-
Targeted Activation of Toll-Like Receptors: Conjugation of a Toll-Like Receptor 7 Agonist to a Monoclonal Antibody Maintains Antigen Binding and Specificity.Bioconjug Chem. 2015 Aug 19;26(8):1743-52. doi: 10.1021/acs.bioconjchem.5b00302. Epub 2015 Jul 16. Bioconjug Chem. 2015. PMID: 26133029
-
Enhancement of the Immunostimulatory Activity of a TLR7 Ligand by Conjugation to Polysaccharides.Bioconjug Chem. 2015 Aug 19;26(8):1713-23. doi: 10.1021/acs.bioconjchem.5b00285. Epub 2015 Aug 5. Bioconjug Chem. 2015. PMID: 26193334
-
Innate immune protection against infectious diseases by pulmonary administration of a phospholipid-conjugated TLR7 ligand.J Innate Immun. 2014;6(3):315-24. doi: 10.1159/000355217. Epub 2013 Nov 1. J Innate Immun. 2014. PMID: 24192551 Free PMC article.
-
Cancer immunotherapeutic potential of novel small molecule TLR7 and TLR8 agonists.J Immunotoxicol. 2009 Dec;6(4):257-65. doi: 10.3109/15476910903286733. J Immunotoxicol. 2009. PMID: 19848448
-
Synthesis and immunoregulatory activities of conjugates of a Toll-like receptor 7 inert ligand.Bioorg Med Chem Lett. 2014 Dec 15;24(24):5792-5795. doi: 10.1016/j.bmcl.2014.10.034. Epub 2014 Oct 17. Bioorg Med Chem Lett. 2014. PMID: 25453821
Cited by
-
Role of human Toll-like receptors in naturally occurring influenza A infections.Influenza Other Respir Viruses. 2013 Sep;7(5):666-75. doi: 10.1111/irv.12109. Epub 2013 Apr 1. Influenza Other Respir Viruses. 2013. PMID: 23552014 Free PMC article.
-
Mast cell-mediated inhibition of abdominal neutrophil inflammation by a PEGylated TLR7 ligand.Mediators Inflamm. 2012;2012:262394. doi: 10.1155/2012/262394. Epub 2011 Nov 17. Mediators Inflamm. 2012. PMID: 22619481 Free PMC article.
-
Protective T cell immunity in mice following protein-TLR7/8 agonist-conjugate immunization requires aggregation, type I IFN, and multiple DC subsets.J Clin Invest. 2011 May;121(5):1782-96. doi: 10.1172/JCI45416. Epub 2011 Apr 11. J Clin Invest. 2011. PMID: 21540549 Free PMC article.
-
Multifunctional Protein Conjugates with Built-in Adjuvant (Adjuvant-Protein-Antigen) as Cancer Vaccines Boost Potent Immune Responses.iScience. 2020 Mar 27;23(3):100935. doi: 10.1016/j.isci.2020.100935. Epub 2020 Feb 24. iScience. 2020. PMID: 32146328 Free PMC article.
-
Selective and potent furin inhibitors protect cells from anthrax without significant toxicity.Int J Biochem Cell Biol. 2010 Jun;42(6):987-95. doi: 10.1016/j.biocel.2010.02.013. Epub 2010 Mar 1. Int J Biochem Cell Biol. 2010. PMID: 20197107 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

