Endogenous IL-12 triggers an antiangiogenic program in melanoma cells
- PMID: 17360466
- PMCID: PMC1820697
- DOI: 10.1073/pnas.0609028104
Endogenous IL-12 triggers an antiangiogenic program in melanoma cells
Abstract
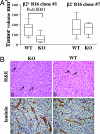
The IL12RB2 gene acts as a tumor suppressor in human B cell malignancies. Indeed, Il12rb2 knockout (KO) mice develop spontaneously B cell tumors, but also lung epithelial tumors. This latter phenotype may be related to (i) impairment of host IL-12-mediated immunosurveillance and/or (ii) IL-12 inability to inhibit directly the growth of IL-12 unresponsive malignant cells. To address this issue, we transplanted IL-12R(+) B16 melanoma cells into syngeneic Il12rb2 KO mice with the following rationale: (i) these mice have severe defects in IFN-gamma production, as well as in cytotoxic T lymphocyte and natural killer cell cytotoxicity, and (ii) they produce but do not use IL-12 that can potentially bind to and target tumor cells only. Il12rb2 KO mice displayed higher endogenous serum levels of IL-12 and developed smaller B16 tumors than WT animals. These tumors showed reduced proliferation, increased apoptosis, and defective microvessel formation related to down-regulated expression of a set of proangiogenic genes previously unrelated to IL-12. Such effects depended on direct activity of endogenous IL-12 on tumor cells in KO mice, and hydrodynamic delivered IL-12 caused further reduced tumorigenicity of B16 cells in these mice. A previously undescribed mechanism of the IL-12 antitumor activity has been here identified and characterized.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
IFN-gamma-inducing factor/IL-18 administration mediates IFN-gamma- and IL-12-independent antitumor effects.J Immunol. 1998 Feb 15;160(4):1742-9. J Immunol. 1998. PMID: 9469432
-
Interleukin-12 elicits a non-canonical response in B16 melanoma cells to enhance survival.Cell Commun Signal. 2020 May 25;18(1):78. doi: 10.1186/s12964-020-00547-4. Cell Commun Signal. 2020. PMID: 32450888 Free PMC article.
-
In vivo elimination of CD25+ regulatory T cells leads to tumor rejection of B16F10 melanoma, when combined with interleukin-12 gene transfer.Exp Dermatol. 2004 Oct;13(10):613-20. doi: 10.1111/j.0906-6705.2004.00198.x. Exp Dermatol. 2004. PMID: 15447721
-
Antitumor effects on mouse melanoma elicited by local secretion of interleukin-12 and their enhancement by treatment with interleukin-18.Cancer Invest. 2000;18(3):206-13. doi: 10.3109/07357900009031825. Cancer Invest. 2000. PMID: 10754989
-
New perspectives for melanoma immunotherapy: role of IL-12.Curr Mol Med. 2009 May;9(4):459-69. doi: 10.2174/156652409788167140. Curr Mol Med. 2009. PMID: 19519403 Review.
Cited by
-
Novel Methylation Patterns Predict Outcome in Uveal Melanoma.Life (Basel). 2020 Oct 20;10(10):248. doi: 10.3390/life10100248. Life (Basel). 2020. PMID: 33092094 Free PMC article.
-
Limited value of pro-inflammatory oxylipins and cytokines as circulating biomarkers in endometriosis - a targeted 'omics study.Sci Rep. 2016 May 19;6:26117. doi: 10.1038/srep26117. Sci Rep. 2016. PMID: 27193963 Free PMC article.
-
IL-12 can target human lung adenocarcinoma cells and normal bronchial epithelial cells surrounding tumor lesions.PLoS One. 2009 Jul 1;4(7):e6119. doi: 10.1371/journal.pone.0006119. PLoS One. 2009. PMID: 19582164 Free PMC article.
-
Tasquinimod triggers an early change in the polarization of tumor associated macrophages in the tumor microenvironment.J Immunother Cancer. 2015 Dec 15;3:53. doi: 10.1186/s40425-015-0098-5. eCollection 2015. J Immunother Cancer. 2015. PMID: 26673090 Free PMC article.
-
Predictive biomarkers for the responsiveness of recurrent glioblastomas to activated killer cell immunotherapy.Cell Biosci. 2023 Jan 24;13(1):17. doi: 10.1186/s13578-023-00961-4. Cell Biosci. 2023. PMID: 36694264 Free PMC article.
References
-
- Trinchieri G. Nat Rev Immunol. 2003;3:133–146. - PubMed
-
- Trinchieri G, Pflanz S, Kastelein RA. Immunity. 2003;19:641–644. - PubMed
-
- Hunter CA. Nat Rev Immunol. 2005;5:521–531. - PubMed
-
- Wolf SF, Temple PA, Kobayashi M, Young D, Dicig M, Lowe L, Dzialo R, Fitz L, Ferenz C, Hewick RM, et al. J Immunol. 1991;146:3074–3081. - PubMed
-
- Chua AO, Chizzonite R, Desai BB, Truitt TP, Nunes P, Minetti LJ, Warrier RR, Presky DH, Levine JF, Gately MK, et al. J Immunol. 1994;153:128–136. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

