Identification of STAT3 as a substrate of receptor protein tyrosine phosphatase T
- PMID: 17360477
- PMCID: PMC1802729
- DOI: 10.1073/pnas.0611665104
Identification of STAT3 as a substrate of receptor protein tyrosine phosphatase T
Abstract
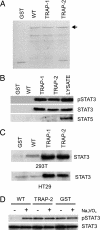
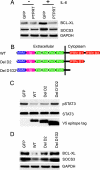
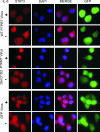
Protein tyrosine phosphatase (PTP) receptor T (PTPRT) is the most frequently mutated PTP in human cancers. However, the cell signaling pathways regulated by PTPRT have not yet been elucidated. Here, we report identification of signal transducer and activator of transcription 3 (STAT3) as a substrate of PTPRT. Phosphorylation of a tyrosine at amino acid Y705 is essential for the function of STAT3, and PTPRT specifically dephosphorylated STAT3 at this position. Accordingly, overexpression of normal PTPRT in colorectal cancer cells reduced the expression of STAT3 target genes. These studies illuminate a mechanism regulating the STAT3 pathway and suggest that this signaling pathway plays an important role in colorectal tumorigenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
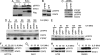
References
-
- Blume-Jensen P, Hunter T. Nature. 2001;411:355–365. - PubMed
-
- Wang Z, Shen D, Parsons DW, Bardelli A, Sager J, Szabo S, Ptak J, Silliman N, Peters BA, van der Heijden MS, et al. Science. 2004;304:1164–1166. - PubMed
-
- Collier LS, Carlson CM, Ravimohan S, Dupuy AJ, Largaespada DA. Nature. 2005;436:272–276. - PubMed
-
- Bowman T, Garcia R, Turkson J, Jove R. Oncogene. 2000;19:2474–2488. - PubMed
-
- Yu H, Jove R. Nat Rev Cancer. 2004;4:97–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

