Steroid receptor coactivator 3 is a coactivator for myocardin, the regulator of smooth muscle transcription and differentiation
- PMID: 17360478
- PMCID: PMC1820709
- DOI: 10.1073/pnas.0611639104
Steroid receptor coactivator 3 is a coactivator for myocardin, the regulator of smooth muscle transcription and differentiation
Abstract
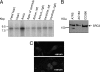
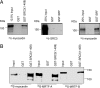
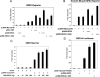
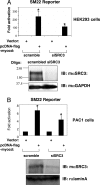
Abnormal proliferation of vascular smooth muscle cells (VSMCs) constitutes a key event in atherosclerosis, neointimal hyperplasia, and the response to vascular injury. Estrogen receptor alpha (ERalpha) mediates the protective effects of estrogen in injured blood vessels and regulates ligand-dependent gene expression in vascular cells. However, the molecular mechanisms mediating ERalpha-dependent VSMC gene expression and VSMC proliferation after vascular injury are not well defined. Here, we report that the ER coactivator steroid receptor coactivator 3 (SRC3) is also a coactivator for the major VSMC transcription factor myocardin, which is required for VSMC differentiation to the nonproliferative, contractile state. The N terminus of SRC3, which contains a basic helix-loop-helix/Per-ARNT-Sim protein-protein interaction domain, binds the C-terminal activation domain of myocardin and enhances myocardin-mediated transcriptional activation of VSMC-specific, CArG-containing promoters, including the VSMC-specific genes SM22 and myosin heavy chain. Suppression of endogenous SRC3 expression by specific small interfering RNA attenuates myocardin transcriptional activation in cultured cells. The SRC3-myocardin interaction identifies a site of convergence for nuclear hormone receptor-mediated and VSMC-specific gene regulation and suggests a possible mechanism for the vascular protective effects of estrogen on vascular injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


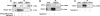
Similar articles
-
Liuwei Dihuang soft capsules inhibits the phenotypic conversion of VSMC to prevent the menopausal atherosclerosis by up-regulating the expression of myocardin.J Ethnopharmacol. 2020 Jan 10;246:112207. doi: 10.1016/j.jep.2019.112207. Epub 2019 Aug 30. J Ethnopharmacol. 2020. PMID: 31476440
-
STAT3 Protein Regulates Vascular Smooth Muscle Cell Phenotypic Switch by Interaction with Myocardin.J Biol Chem. 2015 Aug 7;290(32):19641-52. doi: 10.1074/jbc.M114.630111. Epub 2015 Jun 22. J Biol Chem. 2015. PMID: 26100622 Free PMC article.
-
HERP1 inhibits myocardin-induced vascular smooth muscle cell differentiation by interfering with SRF binding to CArG box.Arterioscler Thromb Vasc Biol. 2005 Nov;25(11):2328-34. doi: 10.1161/01.ATV.0000185829.47163.32. Epub 2005 Sep 8. Arterioscler Thromb Vasc Biol. 2005. PMID: 16151017
-
Myocardin: A novel player in atherosclerosis.Atherosclerosis. 2017 Feb;257:266-278. doi: 10.1016/j.atherosclerosis.2016.12.002. Epub 2016 Dec 1. Atherosclerosis. 2017. PMID: 28012646 Review.
-
Emerging roles of the myocardin family of proteins in lipid and glucose metabolism.J Physiol. 2016 Sep 1;594(17):4741-52. doi: 10.1113/JP271913. Epub 2016 May 10. J Physiol. 2016. PMID: 27060572 Free PMC article. Review.
Cited by
-
Characterization of ASC-2 as an antiatherogenic transcriptional coactivator of liver X receptors in macrophages.Mol Endocrinol. 2009 Jul;23(7):966-74. doi: 10.1210/me.2008-0308. Epub 2009 Apr 2. Mol Endocrinol. 2009. PMID: 19342446 Free PMC article.
-
Predicting tissue-specific gene expression from whole blood transcriptome.Sci Adv. 2021 Apr 2;7(14):eabd6991. doi: 10.1126/sciadv.abd6991. Print 2021 Apr. Sci Adv. 2021. PMID: 33811070 Free PMC article.
-
Relaxin and gonadal steroid receptors in uterosacral ligaments of women with and without pelvic organ prolapse.Int Urogynecol J. 2012 Apr;23(4):495-500. doi: 10.1007/s00192-011-1615-9. Epub 2011 Nov 29. Int Urogynecol J. 2012. PMID: 22124513
-
1,25-dihydroxyvitamin D3 treatment shrinks uterine leiomyoma tumors in the Eker rat model.Biol Reprod. 2012 Apr 19;86(4):116. doi: 10.1095/biolreprod.111.098145. Print 2012 Apr. Biol Reprod. 2012. PMID: 22302692 Free PMC article.
-
Nexilin/NEXN controls actin polymerization in smooth muscle and is regulated by myocardin family coactivators and YAP.Sci Rep. 2018 Aug 29;8(1):13025. doi: 10.1038/s41598-018-31328-2. Sci Rep. 2018. PMID: 30158653 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

