Antitumor activity of MLN8054, an orally active small-molecule inhibitor of Aurora A kinase
- PMID: 17360485
- PMCID: PMC1820716
- DOI: 10.1073/pnas.0608798104
Antitumor activity of MLN8054, an orally active small-molecule inhibitor of Aurora A kinase
Abstract
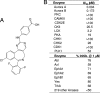
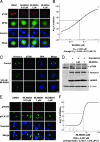
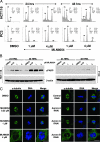
Increased Aurora A expression occurs in a variety of human cancers and induces chromosomal abnormalities during mitosis associated with tumor initiation and progression. MLN8054 is a selective small-molecule Aurora A kinase inhibitor that has entered Phase I clinical trials for advanced solid tumors. MLN8054 inhibits recombinant Aurora A kinase activity in vitro and is selective for Aurora A over the family member Aurora B in cultured cells. MLN8054 treatment results in G(2)/M accumulation and spindle defects and inhibits proliferation in multiple cultured human tumor cells lines. Growth of human tumor xenografts in nude mice was dramatically inhibited after oral administration of MLN8054 at well tolerated doses. Moreover, the tumor growth inhibition was sustained after discontinuing MLN8054 treatment. In human tumor xenografts, MLN8054 induced mitotic accumulation and apoptosis, phenotypes consistent with inhibition of Aurora A. MLN8054 is a selective inhibitor of Aurora A kinase that robustly inhibits growth of human tumor xenografts and represents an attractive modality for therapeutic intervention of human cancers.
Conflict of interest statement
Conflict of interest statement: The authors of this paper, who are employees of Millennium Pharmaceuticals, Inc., are stock holders in the company.
Figures
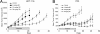

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases
Miscellaneous

