The proton pumping pathway of bovine heart cytochrome c oxidase
- PMID: 17360500
- PMCID: PMC1820732
- DOI: 10.1073/pnas.0611627104
The proton pumping pathway of bovine heart cytochrome c oxidase
Abstract
X-ray structures of bovine heart cytochrome c oxidase have suggested that the enzyme, which reduces O(2) in a process coupled with a proton pumping process, contains a proton pumping pathway (H-pathway) composed of a hydrogen bond network and a water channel located in tandem across the enzyme. The hydrogen bond network includes the peptide bond between Tyr-440 and Ser-441, which could facilitate unidirectional proton transfer. Replacement of a possible proton-ejecting aspartate (Asp-51) at one end of the H-pathway with asparagine, using a stable bovine gene expression system, abolishes the proton pumping activity without influencing the O(2) reduction function. Blockage of either the water channel by a double mutation (Val386Leu and Met390Trp) or proton transfer through the peptide by a Ser441Pro mutation was found to abolish the proton pumping activity without impairment of the O(2) reduction activity. These results significantly strengthen the proposal that H-pathway is involved in proton pumping.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


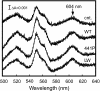
 C(OH)
C(OH) N+H
N+H ) (8), followed by removal of the imidic acid proton by D51 to form the enol tautomer of the peptide (
) (8), followed by removal of the imidic acid proton by D51 to form the enol tautomer of the peptide ( C(OH)
C(OH) N
N ) (6). The enol form is then tautomerized back to the keto form (
) (6). The enol form is then tautomerized back to the keto form ( CO-NH
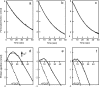
CO-NH ) because the latter is more stable. (b) Protonation of the peptide bond by proton from the negative side in the Ser441Pro mutant. (c) Prediction of the conformation of the Ser441Pro mutant. The purple structures show the conformational changes of the oxidized form induced by the Ser441Pro mutation as predicted by X-PLOR analysis. The dark blue structure denotes the conformational change occurring upon reduction of the enzyme, wherein no influence by the mutation is detectable. The red dotted lines indicate hydrogen bonds, and the red, yellow, and light blue structures denote oxygen, carbon, and nitrogen atoms, respectively.
) because the latter is more stable. (b) Protonation of the peptide bond by proton from the negative side in the Ser441Pro mutant. (c) Prediction of the conformation of the Ser441Pro mutant. The purple structures show the conformational changes of the oxidized form induced by the Ser441Pro mutation as predicted by X-PLOR analysis. The dark blue structure denotes the conformational change occurring upon reduction of the enzyme, wherein no influence by the mutation is detectable. The red dotted lines indicate hydrogen bonds, and the red, yellow, and light blue structures denote oxygen, carbon, and nitrogen atoms, respectively.

References
-
- Iwata S, Ostermeier C, Ludwig B, Michel H. Nature. 1995;376:660–669. - PubMed
-
- Tsukihara T, Aoyama H, Yamashita E, Tomizaki T, Yamaguchi H, Shinzawa-Itoh K, Nakashima R, Yaono R, Yoshikawa S. Science. 1996;272:1136–1144. - PubMed
-
- Yoshikawa S, Shinzawa-Itoh K, Nakashima R, Yaono R, Yamashita E, Inoue N, Yao M, Fei MJ, Libeu CP, Mizushima T, et al. Science. 1998;280:1723–1729. - PubMed
-
- Svensson-Ek M, Abramson J, Larsson G, Tornroth S, Brzezinski P, Iwata S. J Mol Biol. 2002;321:329–339. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

