Urocortin 3 regulates glucose-stimulated insulin secretion and energy homeostasis
- PMID: 17360501
- PMCID: PMC1820733
- DOI: 10.1073/pnas.0611641104
Urocortin 3 regulates glucose-stimulated insulin secretion and energy homeostasis
Erratum in
- Proc Natl Acad Sci U S A. 2008 May 13;105(19):7106
Abstract
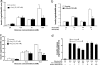
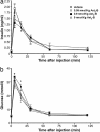
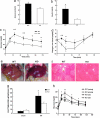
Urocortin 3 (Ucn 3), a member of the corticotropin-releasing factor (CRF) family of peptides, is strongly expressed in mammalian pancreatic beta cells and has been shown to stimulate insulin secretion. Here we report the investigation of the hypothesis that endogenous Ucn 3 regulates insulin secretion, particularly in the presence of nutrient excess. Secretion of Ucn 3-like immunoreactivity from cultured beta cells was stimulated by high glucose and insulin secretagogs such as GLP-1; furthermore, 5 pancreatic Ucn 3 mRNA levels in vivo were increased during the positive energy balance caused by high-fat diet and by the absence of leptin. Immunoneutralization of Ucn 3 or pharmacologic blockade of its receptor, the type 2 CRF receptor (CRFR2), attenuated high but not low glucose-induced insulin secretion from isolated islets in vitro. Cultured islets isolated from Ucn 3-null mice also secreted less insulin in response to high glucose concentrations. Consistently, peripheral injection of a selective CRFR2 antagonist before the administration of a glucose challenge significantly attenuated glucose-induced insulin secretion in vivo. Ucn 3-null mice were relatively protected from the hyperinsulinemia, hyperglycemia, glucose intolerance, hepatic steatosis, and hypertriglyceridemia induced by high-fat diet. Additionally, we found that aged Ucn 3-null mice maintained better glucose tolerance than age-matched wild-type littermates. These results suggest that endogenous Ucn 3 in the pancreas is induced under excessive caloric conditions and acts locally to augment insulin production, which in the long-term may contribute to reduced insulin sensitivity and harmful metabolic consequences.
Conflict of interest statement
Conflict of interest statement: W.V. is a cofounder, consultant, equity holder, and member of the Board of Directors of Neurocrine Biosciences and Acceleron Pharma. The following have been licensed by the Salk Institute and/or Clayton Foundation: CRF to Ferring Pharmaceuticals and CRF1 receptor to Neurocrine Biosciences.
Figures
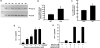

References
-
- Hsu SY, Hsueh AJ. Nat Med. 2001;7:605–611. - PubMed
-
- Li C, Chen P, Vaughan J, Blount A, Chen A, Jamieson PM, Rivier J, Smith MS, Vale W. Endocrinology. 2003;144:3216–3224. - PubMed
-
- Kanno T, Suga S, Nakano K, Kamimura N, Wakui M. Diabetes. 1999;48:1741–1746. - PubMed
-
- Drucker DJ. Cell Metab. 2006;3:153–165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

