Chemical genetics reveals the requirement for Polo-like kinase 1 activity in positioning RhoA and triggering cytokinesis in human cells
- PMID: 17360533
- PMCID: PMC1838611
- DOI: 10.1073/pnas.0701140104
Chemical genetics reveals the requirement for Polo-like kinase 1 activity in positioning RhoA and triggering cytokinesis in human cells
Abstract
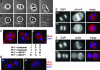
Polo-like kinases (Plks) play crucial roles in mitosis and cell division. Whereas lower eukaryotes typically contain a single Plk, mammalian cells express several closely related but functionally distinct Plks. We describe here a chemical genetic system in which a single Plk family member, Plk1, can be inactivated with high selectivity and temporal resolution by using an allele-specific, small-molecule inhibitor, as well as the application of this system to dissect Plk1's role in cytokinesis. To do this, we disrupted both copies of the PLK1 locus in human cells through homologous recombination and then reconstituted Plk1 activity by using either the wild-type kinase (Plk1(wt)) or a mutant version whose catalytic pocket has been enlarged to accommodate bulky purine analogs (Plk1(as)). When cultured in the presence of these analogs, Plk1(as) cells accumulate in prometaphase with defects that parallel those found in PLK1(Delta/Delta) cells. In addition, acute treatment of Plk1(as) cells during anaphase prevents recruitment of both Plk1 itself and the Rho guanine nucleotide exchange factor (RhoGEF) Ect2 to the central spindle, abolishes RhoA GTPase localization to the equatorial cortex, and suppresses cleavage furrow formation and cell division. Our studies define and illuminate a late mitotic function of Plk1 that, although difficult or impossible to detect in Plk1-depleted cells, is readily revealed with chemical genetics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

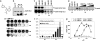


References
-
- van de Weerdt BC, Medema RH. Cell Cycle. 2006;5:853–864. - PubMed
-
- Barr FA, Sillje HH, Nigg EA. Nat Rev Mol Cell Biol. 2004;5:429–441. - PubMed
-
- Peters U, Cherian J, Kim JH, Kwok BH, Kapoor TM. Nat Chem Biol. 2006;2:618–626. - PubMed
-
- McInnes C, Mazumdar A, Mezna M, Meades C, Midgley C, Scaerou F, Carpenter L, Mackenzie M, Taylor P, Walkinshaw M, et al. Nat Chem Biol. 2006;2:608–617. - PubMed
-
- Liu Y, Shreder KR, Gai W, Corral S, Ferris DK, Rosenblum JS. Chem Biol. 2005;12:99–107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

