RBP-J (Rbpsuh) is essential to maintain muscle progenitor cells and to generate satellite cells
- PMID: 17360543
- PMCID: PMC1815471
- DOI: 10.1073/pnas.0610647104
RBP-J (Rbpsuh) is essential to maintain muscle progenitor cells and to generate satellite cells
Abstract
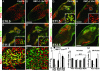
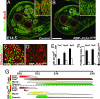
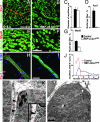
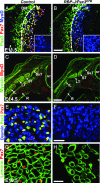
In the developing muscle, a pool of myogenic progenitor cells is formed and maintained. These resident progenitors provide a source of cells for muscle growth in development and generate satellite cells in the perinatal period. By the use of conditional mutagenesis in mice, we demonstrate here that the major mediator of Notch signaling, the transcription factor RBP-J, is essential to maintain this pool of progenitor cells in an undifferentiated state. In the absence of RBP-J, these cells undergo uncontrolled myogenic differentiation, leading to a depletion of the progenitor pool. This results in a lack of muscle growth in development and severe muscle hypotrophy. In addition, satellite cells are not formed late in fetal development in conditional RBP-J mutant mice. We conclude that RBP-J is required in the developing muscle to set aside proliferating progenitors and satellite cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Buckingham M. Curr Opin Genet Dev. 2006;16:525–532. - PubMed
-
- Arnold HH, Braun T. Curr Top Dev Biol. 2000;48:129–164. - PubMed
-
- Buckingham M. Curr Opin Genet Dev. 2001;11:440–448. - PubMed
-
- Parker MH, Seale P, Rudnicki MA. Nat Rev Genet. 2003;4:497–507. - PubMed
-
- Ordahl CP, Le Douarin NM. Development (Cambridge, UK) 1992;114:339–353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

