A pure population of lung alveolar epithelial type II cells derived from human embryonic stem cells
- PMID: 17360544
- PMCID: PMC1838621
- DOI: 10.1073/pnas.0700052104
A pure population of lung alveolar epithelial type II cells derived from human embryonic stem cells
Abstract
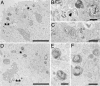
Alveolar epithelial type II (ATII) cells are small, cuboidal cells that constitute approximately 60% of the pulmonary alveolar epithelium. These cells are crucial for repair of the injured alveolus by differentiating into alveolar epithelial type I cells. ATII cells derived from human ES (hES) cells are a promising source of cells that could be used therapeutically to treat distal lung diseases. We have developed a reliable transfection and culture procedure, which facilitates, via genetic selection, the differentiation of hES cells into an essentially pure (>99%) population of ATII cells (hES-ATII). Purity, as well as biological features and morphological characteristics of normal ATII cells, was demonstrated for the hES-ATII cells, including lamellar body formation, expression of surfactant proteins A, B, and C, alpha-1-antitrypsin, and the cystic fibrosis transmembrane conductance receptor, as well as the synthesis and secretion of complement proteins C3 and C5. Collectively, these data document the successful generation of a pure population of ATII cells derived from hES cells, providing a practical source of ATII cells to explore in disease models their potential in the regeneration and repair of the injured alveolus and in the therapeutic treatment of genetic diseases affecting the lung.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Rothman BL, Despins AW, Kreutzer DL. J Immunol. 1990;145:592–598. - PubMed
-
- Zhao YX, Andoh A, Shimada M, Takaya H, Hata K, Fujiyama Y, Bamda T. Int J Mol Med. 2000;5:415–419. - PubMed
-
- Mason RJ. Respirology. 2006;11(Suppl):S12–S15. - PubMed
-
- Nogee LM, de Mello DE, Dehner LP, Colten HR. N Engl J Med. 1993;328:406–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

