Importance of culturing primary lymphocytes at physiological oxygen levels
- PMID: 17360561
- PMCID: PMC1838638
- DOI: 10.1073/pnas.0611732104
Importance of culturing primary lymphocytes at physiological oxygen levels
Abstract
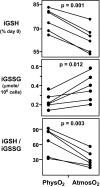
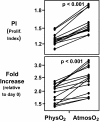
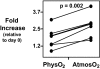
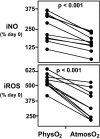
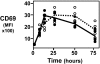
Although studies with primary lymphocytes are almost always conducted in CO(2) incubators maintained at atmospheric oxygen levels (atmosO(2); 20%), the physiological oxygen levels (physO(2); 5%) that cells encounter in vivo are 2-4 times lower. We show here that culturing primary T cells at atmosO(2) significantly alters the intracellular redox state (decreases intracellular glutathione, increases oxidized intracellular glutathione), whereas culturing at physO(2) maintains the intracellular redox environment (intracellular glutathione/oxidized intracellular glutathione) close to its in vivo status. Furthermore, we show that CD3/CD28-induced T cell proliferation (based on proliferation index and cell yield) is higher at atmosO(2) than at physO(2). This apparently paradoxical finding, we suggest, may be explained by two additional findings with CD3/CD28-stimulated T cells: (i) the intracellular NO (iNO) levels are higher at physO(2) than at atmosO(2); and (ii) the peak expression of CD69 is significantly delayed and more sustained at physO(2) that at atmosO(2). Because high levels of intracellular NO and sustained CD69 tend to down-regulate T cell responses in vivo, the lower proliferative T cell responses at physO(2) likely reflect the in vitro operation of the natural in vivo regulatory mechanisms. Thus, we suggest caution in culturing primary lymphocytes at atmosO(2) because the requisite adaptation to nonphysiological oxygen levels may seriously skew T cell responses, particularly after several days in culture.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

