Impaired angiogenesis in aminopeptidase N-null mice
- PMID: 17360568
- PMCID: PMC1815469
- DOI: 10.1073/pnas.0611653104
Impaired angiogenesis in aminopeptidase N-null mice
Abstract
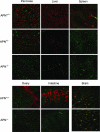
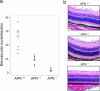
Aminopeptidase N (APN, CD13; EC 3.4.11.2) is a transmembrane metalloprotease with several functions, depending on the cell type and tissue environment. In tumor vasculature, APN is overexpressed in the endothelium and promotes angiogenesis. However, there have been no reports of in vivo inactivation of the APN gene to validate these findings. Here we evaluated, by targeted disruption of the APN gene, whether APN participates in blood vessel formation and function under normal conditions. Surprisingly, APN-null mice developed with no gross or histological abnormalities. Standard neurological, cardiovascular, metabolic, locomotor, and hematological studies revealed no alterations. Nonetheless, in oxygen-induced retinopathy experiments, APN-deficient mice had a marked and dose-dependent deficiency of the expected retinal neovascularization. Moreover, gelfoams embedded with growth factors failed to induce functional blood vessel formation in APN-null mice. These findings establish that APN-null mice develop normally without physiological alterations and can undergo physiological angiogenesis but show a severely impaired angiogenic response under pathological conditions. Finally, in addition to vascular biology research, APN-null mice may be useful reagents in other medical fields such as malignant, cardiovascular, immunological, or infectious diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


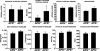
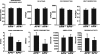
Similar articles
-
The angiogenic regulator CD13/APN is a transcriptional target of Ras signaling pathways in endothelial morphogenesis.Blood. 2003 Mar 1;101(5):1818-26. doi: 10.1182/blood-2002-05-1422. Epub 2002 Oct 24. Blood. 2003. PMID: 12406907
-
CD13/APN is activated by angiogenic signals and is essential for capillary tube formation.Blood. 2001 Feb 1;97(3):652-9. doi: 10.1182/blood.v97.3.652. Blood. 2001. PMID: 11157481 Free PMC article.
-
Cooperative effects of aminopeptidase N (CD13) expressed by nonmalignant and cancer cells within the tumor microenvironment.Proc Natl Acad Sci U S A. 2012 Jan 31;109(5):1637-42. doi: 10.1073/pnas.1120790109. Epub 2012 Jan 17. Proc Natl Acad Sci U S A. 2012. PMID: 22307623 Free PMC article.
-
The structure and main functions of aminopeptidase N.Curr Med Chem. 2007;14(6):639-47. doi: 10.2174/092986707780059571. Curr Med Chem. 2007. PMID: 17346152 Review.
-
Aminopeptidase N (CD13) as a target for cancer chemotherapy.Cancer Sci. 2011 Mar;102(3):501-8. doi: 10.1111/j.1349-7006.2010.01826.x. Epub 2011 Jan 30. Cancer Sci. 2011. PMID: 21205077 Free PMC article. Review.
Cited by
-
Pericytes, microvasular dysfunction, and chronic rejection.Transplantation. 2015 Apr;99(4):658-67. doi: 10.1097/TP.0000000000000648. Transplantation. 2015. PMID: 25793439 Free PMC article. Review.
-
Aminopeptidase activities as prospective urinary biomarkers for bladder cancer.Proteomics Clin Appl. 2014 Jun;8(5-6):317-26. doi: 10.1002/prca.201300118. Epub 2014 Mar 31. Proteomics Clin Appl. 2014. PMID: 24591208 Free PMC article.
-
Sustained release of endothelial progenitor cell-derived extracellular vesicles from shear-thinning hydrogels improves angiogenesis and promotes function after myocardial infarction.Cardiovasc Res. 2018 Jun 1;114(7):1029-1040. doi: 10.1093/cvr/cvy067. Cardiovasc Res. 2018. PMID: 29566124 Free PMC article.
-
CD13-positive bone marrow-derived myeloid cells promote angiogenesis, tumor growth, and metastasis.Proc Natl Acad Sci U S A. 2013 Dec 17;110(51):20717-22. doi: 10.1073/pnas.1321139110. Epub 2013 Dec 2. Proc Natl Acad Sci U S A. 2013. PMID: 24297924 Free PMC article.
-
Identification of alanyl aminopeptidase (CD13) as a surface marker for isolation of mature gastric zymogenic chief cells.Am J Physiol Gastrointest Liver Physiol. 2015 Dec 15;309(12):G955-64. doi: 10.1152/ajpgi.00261.2015. Epub 2015 Oct 29. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 26514774 Free PMC article.
References
-
- Hooper NM, Lendeckel U, editors. Aminopeptidases in Biology and Disease. New York: Kluwer Academic/Plenum; 2004.
-
- Barrett AJ, Rawlings ND, Woessner JF, editors. Handbook of Proteolytic Enzymes. London: Elsevier; 2004.
-
- Amoscato AA, Alexander JW, Babcock GF. J Immunol. 1989;142:1245–1252. - PubMed
-
- Favaloro EJ, Bradstock KF, Kabral A, Grimsley P, Zowtyj H, Zola H. Br J Haematol. 1988;69:163–171. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

