Pleiotrophin is a neurotrophic factor for spinal motor neurons
- PMID: 17360581
- PMCID: PMC1838658
- DOI: 10.1073/pnas.0603243104
Pleiotrophin is a neurotrophic factor for spinal motor neurons
Abstract
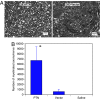
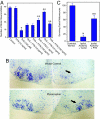
Regeneration in the peripheral nervous system is poor after chronic denervation. Denervated Schwann cells act as a "transient target" by secreting growth factors to promote regeneration of axons but lose this ability with chronic denervation. We discovered that the mRNA for pleiotrophin (PTN) was highly up-regulated in acutely denervated distal sciatic nerves, but high levels of PTN mRNA were not maintained in chronically denervated nerves. PTN protected spinal motor neurons against chronic excitotoxic injury and caused increased outgrowth of motor axons out of the spinal cord explants and formation of "miniventral rootlets." In neonatal mice, PTN protected the facial motor neurons against cell death induced by deprivation from target-derived growth factors. Similarly, PTN significantly enhanced regeneration of myelinated axons across a graft in the transected sciatic nerve of adult rats. Our findings suggest a neurotrophic role for PTN that may lead to previously unrecognized treatment options for motor neuron disease and motor axonal regeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

