Activation of the subventricular zone in multiple sclerosis: evidence for early glial progenitors
- PMID: 17360586
- PMCID: PMC3025281
- DOI: 10.1073/pnas.0606835104
Activation of the subventricular zone in multiple sclerosis: evidence for early glial progenitors
Abstract
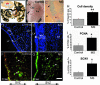
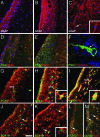
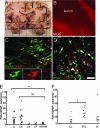
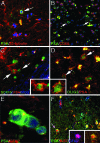
In multiple sclerosis (MS), oligodendrocyte and myelin destruction lead to demyelination with subsequent axonal loss. Experimental demyelination in rodents has highlighted the activation of the subventricular zone (SVZ) and the involvement of progenitor cells expressing the polysialylated form of neural cell adhesion molecule (PSA-NCAM) in the repair process. In this article, we studied the distribution of early PSA-NCAM(+) progenitors in the SVZ and MS lesions in human postmortem brains. Compared with controls, MS SVZ showed a 2- to 3-fold increase in cell density and proliferation, which correlated with enhanced numbers of PSA-NCAM(+) and glial fibrillary acidic protein-positive (GFAP(+)) cells. PSA-NCAM(+) progenitors mainly were Sox9(+), and a few expressed Sox10 and Olig2, markers of oligodendroglial specification. PSA-NCAM(+) progenitors expressing Sox10 and Olig2 also were detected in demyelinated MS lesions. In active and chronic active lesions, the number of PSA-NCAM(+) progenitors was 8-fold higher compared with chronic silent lesions, shadow plaques, and normal-appearing white matter. In active and chronic active lesions, PSA-NCAM(+) progenitors were more frequent in periventricular lesions (30-50%) than in lesions remote from the ventricular wall. These data indicate that, as in rodents, activation of gliogenesis in the SVZ occurs in MS and suggest the mobilization of SVZ-derived early glial progenitors to periventricular lesions, where they could give rise to oligodendrocyte precursors. These early glial progenitors could be a potential target for therapeutic strategies designed to promote myelin repair in MS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The role of SVZ-derived neural precursors in demyelinating diseases: from animal models to multiple sclerosis.J Neurol Sci. 2008 Feb 15;265(1-2):26-31. doi: 10.1016/j.jns.2007.09.032. Epub 2007 Oct 24. J Neurol Sci. 2008. PMID: 17961598 Review.
-
Glial fibrillary acidic protein-expressing neural progenitors give rise to immature neurons via early intermediate progenitors expressing both glial fibrillary acidic protein and neuronal markers in the adult hippocampus.Neuroscience. 2010 Mar 10;166(1):241-51. doi: 10.1016/j.neuroscience.2009.12.026. Epub 2009 Dec 16. Neuroscience. 2010. PMID: 20026190
-
Sox9 and Sox10 influence survival and migration of oligodendrocyte precursors in the spinal cord by regulating PDGF receptor alpha expression.Development. 2008 Feb;135(4):637-46. doi: 10.1242/dev.010454. Epub 2008 Jan 9. Development. 2008. PMID: 18184726
-
Evidence that nucleocytoplasmic Olig2 translocation mediates brain-injury-induced differentiation of glial precursors to astrocytes.J Neurosci Res. 2007 Aug 1;85(10):2126-37. doi: 10.1002/jnr.21368. J Neurosci Res. 2007. PMID: 17510983
-
Untangling the functional potential of PSA-NCAM-expressing cells in CNS development and brain repair strategies.Curr Med Chem. 2003 Oct;10(20):2185-96. doi: 10.2174/0929867033456774. Curr Med Chem. 2003. PMID: 12871092 Review.
Cited by
-
Dibutyryl cyclic AMP inhibits the progression of experimental autoimmune encephalomyelitis and potentiates recruitment of endogenous neural stem cells.J Mol Neurosci. 2013 Oct;51(2):298-306. doi: 10.1007/s12031-013-9959-x. Epub 2013 Jan 19. J Mol Neurosci. 2013. PMID: 23335001
-
Anti-inflammatory treatment induced regenerative oligodendrogenesis in parkinsonian mice.Stem Cell Res Ther. 2012 Aug 14;3(4):33. doi: 10.1186/scrt124. Stem Cell Res Ther. 2012. PMID: 22892385 Free PMC article.
-
Bone Morphogenetic Protein 4 Signalling in Neural Stem and Progenitor Cells during Development and after Injury.Stem Cells Int. 2016;2016:9260592. doi: 10.1155/2016/9260592. Epub 2016 May 16. Stem Cells Int. 2016. PMID: 27293450 Free PMC article. Review.
-
Antiretroviral treatment reveals a novel role for lysosomes in oligodendrocyte maturation.J Neurochem. 2023 Jun;165(5):722-740. doi: 10.1111/jnc.15773. Epub 2023 Feb 16. J Neurochem. 2023. PMID: 36718947 Free PMC article.
-
The role of neural stem cells in regulating glial scar formation and repair.Cell Tissue Res. 2022 Mar;387(3):399-414. doi: 10.1007/s00441-021-03554-0. Epub 2021 Nov 25. Cell Tissue Res. 2022. PMID: 34820704 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

