Accumulation of prion protein in the brain that is not associated with transmissible disease
- PMID: 17360589
- PMCID: PMC1838665
- DOI: 10.1073/pnas.0609241104
Accumulation of prion protein in the brain that is not associated with transmissible disease
Abstract
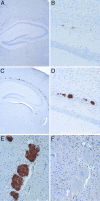
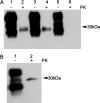
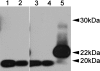
Prion diseases or transmissible spongiform encephalopathies are characterized histopathologically by the accumulation of prion protein (PrP) ranging from diffuse deposits to amyloid plaques. Moreover, pathologic PrP isoforms (PrP(Sc)) are detected by immunoblot analysis and used both as diagnostic markers of disease and as indicators of the presence of infectivity in tissues. It is not known which forms of PrP are associated with infectivity. To address this question, we performed bioassays using human brain extracts from two cases with phenotypically distinct forms of familial prion disease (Gerstmann-Sträussler-Scheinker P102L). Both cases had PrP accumulations in the brain, but each had different PrP(Sc) isoforms. Only one of the brains had spongiform degeneration. Tissue from this case transmitted disease efficiently to transgenic mice (Tg PrP101LL), resulting in spongiform encephalopathy. In contrast, inoculation of tissue from the case with no spongiform degeneration resulted in almost complete absence of disease transmission but elicited striking PrP-amyloid deposition in several recipient mouse brains. Brains of these mice failed to transmit any neurological disease on passage, but PrP-amyloid deposition was again observed in the brains of recipient mice. These data suggest the possible isolation of an infectious agent that promotes PrP amyloidogenesis in the absence of a spongiform encephalopathy. Alternatively, the infectious agent may be rendered nonpathogenic by sequestration in amyloid plaques, or PrP amyloid can seed amyloid accumulation in the brain, causing a proteinopathy that is unrelated to prion disease. Formation of PrP amyloid may therefore not necessarily be a reliable marker of transmissible spongiform encephalopathy infectivity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
PrP aggregation can be seeded by pre-formed recombinant PrP amyloid fibrils without the replication of infectious prions.Acta Neuropathol. 2016 Oct;132(4):611-24. doi: 10.1007/s00401-016-1594-5. Epub 2016 Jul 4. Acta Neuropathol. 2016. PMID: 27376534 Free PMC article.
-
Dissociation of prion protein amyloid seeding from transmission of a spongiform encephalopathy.J Virol. 2013 Nov;87(22):12349-56. doi: 10.1128/JVI.00673-13. Epub 2013 Sep 11. J Virol. 2013. PMID: 24027305 Free PMC article.
-
Mechanism of PrP-amyloid formation in mice without transmissible spongiform encephalopathy.Brain Pathol. 2012 Jan;22(1):58-66. doi: 10.1111/j.1750-3639.2011.00508.x. Epub 2011 Jul 25. Brain Pathol. 2012. PMID: 21645162 Free PMC article.
-
Genetic and infectious prion diseases.Arch Neurol. 1993 Nov;50(11):1129-53. doi: 10.1001/archneur.1993.00540110011002. Arch Neurol. 1993. PMID: 8105771 Review.
-
Prion encephalopathies of animals and humans.Dev Biol Stand. 1993;80:31-44. Dev Biol Stand. 1993. PMID: 8270114 Review.
Cited by
-
Evidence of in utero transmission of classical scrapie in sheep.J Virol. 2014 Apr;88(8):4591-4. doi: 10.1128/JVI.03264-13. Epub 2014 Jan 22. J Virol. 2014. PMID: 24453368 Free PMC article.
-
Selective vulnerability to neurodegenerative disease: the curious case of Prion Protein.Dis Model Mech. 2014 Jan;7(1):21-9. doi: 10.1242/dmm.012146. Dis Model Mech. 2014. PMID: 24396151 Free PMC article. Review.
-
Atypical and classical forms of the disease-associated state of the prion protein exhibit distinct neuronal tropism, deposition patterns, and lesion profiles.Am J Pathol. 2013 Nov;183(5):1539-1547. doi: 10.1016/j.ajpath.2013.07.024. Epub 2013 Sep 5. Am J Pathol. 2013. PMID: 24012784 Free PMC article.
-
Transmissibility of Gerstmann-Sträussler-Scheinker syndrome in rodent models: New insights into the molecular underpinnings of prion infectivity.Prion. 2016 Nov;10(6):421-433. doi: 10.1080/19336896.2016.1239686. Prion. 2016. PMID: 27892798 Free PMC article. Review.
-
Gerstmann-Sträussler-Scheinker disease subtypes efficiently transmit in bank voles as genuine prion diseases.Sci Rep. 2016 Feb 4;6:20443. doi: 10.1038/srep20443. Sci Rep. 2016. PMID: 26841849 Free PMC article.
References
-
- Prusiner SB, editor. Prion Biology and Diseases. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 2004. pp. 89–141.
-
- Collinge J. Annu Rev Neurosci. 2001;24:519–550. - PubMed
-
- Ghetti B, Piccardo P, Frangione B, Bugiani O, Giaccone G, Young K, Prelli F, Farlow MR, Dlouhy SR, Tagliavini F. Brain Pathol. 1996;6:127–145. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

