Fusicoccins are biosynthesized by an unusual chimera diterpene synthase in fungi
- PMID: 17360612
- PMCID: PMC1805559
- DOI: 10.1073/pnas.0608426104
Fusicoccins are biosynthesized by an unusual chimera diterpene synthase in fungi
Abstract
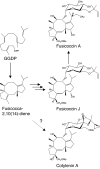
Fusicoccins are a class of diterpene glucosides produced by the plant-pathogenic fungus Phomopsis amygdali. As modulators of 14-3-3 proteins, fusicoccins function as potent activators of plasma membrane H(+)-ATPase in plants and also exhibit unique biological activity in animal cells. Despite their well studied biological activities, no genes encoding fusicoccin biosynthetic enzymes have been identified. Cyclic diterpenes are commonly synthesized via cyclization of a C(20) precursor, geranylgeranyl diphosphate (GGDP), which is produced through condensation of the universal C(5) isoprene units dimethylallyl diphosphate and isopentenyl diphosphate by prenyltransferases. We found that (+)-fusicocca-2,10 (14)-diene, a tricyclic hydrocarbon precursor for fusicoccins, is biosynthesized from the C(5) isoprene units by an unusual multifunctional enzyme, P. amygdali fusicoccadiene synthase (PaFS), which shows both prenyltransferase and terpene cyclase activities. The functional analysis of truncated mutants and site-directed mutagenesis demonstrated that PaFS consists of two domains: a terpene cyclase domain at the N terminus and a prenyltransferase domain at the C terminus. These findings suggest that fusicoccadiene can be produced efficiently in the fungus by using the C(5) precursors, irrespective of GGDP availability. In fact, heterologous expression of PaFS alone resulted in the accumulation of fusicocca-2,10 (14)-diene in Escherichia coli cells, whereas no product was detected in E. coli cells expressing Gibberella fujikuroi ent-kaurene synthase, another fungal diterpene cyclase that also uses GGDP as a substrate but does not contain a prenyltransferase domain. Genome walking suggested that fusicoccin biosynthetic enzymes are encoded as a gene cluster near the PaFS gene.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Recent advances regarding diterpene cyclase genes in higher plants and fungi.Biosci Biotechnol Biochem. 2008 May;72(5):1168-75. doi: 10.1271/bbb.80044. Epub 2008 May 7. Biosci Biotechnol Biochem. 2008. PMID: 18460786 Review.
-
Structural insight on assembly-line catalysis in terpene biosynthesis.Nat Commun. 2021 Jun 9;12(1):3487. doi: 10.1038/s41467-021-23589-9. Nat Commun. 2021. PMID: 34108468 Free PMC article.
-
Transient Prenyltransferase-Cyclase Association in Fusicoccadiene Synthase, an Assembly-Line Terpene Synthase.Biochemistry. 2022 Nov 1;61(21):2417-2430. doi: 10.1021/acs.biochem.2c00509. Epub 2022 Oct 13. Biochemistry. 2022. PMID: 36227241 Free PMC article.
-
Methods for the preparation and analysis of the diterpene cyclase fusicoccadiene synthase.Methods Enzymol. 2024;699:89-119. doi: 10.1016/bs.mie.2023.11.003. Epub 2023 Dec 8. Methods Enzymol. 2024. PMID: 38942517 Free PMC article.
-
Assembly-Line Catalysis in Bifunctional Terpene Synthases.Acc Chem Res. 2021 Oct 19;54(20):3780-3791. doi: 10.1021/acs.accounts.1c00296. Epub 2021 Jul 13. Acc Chem Res. 2021. PMID: 34254507 Free PMC article. Review.
Cited by
-
Schultriene and nigtetraene: two sesterterpenes characterized from pathogenetic fungi via genome mining approach.Appl Microbiol Biotechnol. 2022 Sep;106(18):6047-6057. doi: 10.1007/s00253-022-12125-4. Epub 2022 Aug 30. Appl Microbiol Biotechnol. 2022. PMID: 36040489
-
Multi-domain terpenoid cyclase architecture and prospects for proximity in bifunctional catalysis.Curr Opin Struct Biol. 2016 Dec;41:27-37. doi: 10.1016/j.sbi.2016.05.010. Epub 2016 Jun 7. Curr Opin Struct Biol. 2016. PMID: 27285057 Free PMC article. Review.
-
Discovery of non-squalene triterpenes.Nature. 2022 Jun;606(7913):414-419. doi: 10.1038/s41586-022-04773-3. Epub 2022 Jun 1. Nature. 2022. PMID: 35650436 Free PMC article.
-
Functional characterisation of twelve terpene synthases from actinobacteria.Beilstein J Org Chem. 2023 Sep 15;19:1386-1398. doi: 10.3762/bjoc.19.100. eCollection 2023. Beilstein J Org Chem. 2023. PMID: 37736393 Free PMC article.
-
Cloning and characterization of an Armillaria gallica cDNA encoding protoilludene synthase, which catalyzes the first committed step in the synthesis of antimicrobial melleolides.J Biol Chem. 2011 Mar 4;286(9):6871-8. doi: 10.1074/jbc.M110.165845. Epub 2010 Dec 10. J Biol Chem. 2011. PMID: 21148562 Free PMC article.
References
-
- Ballio A, Brufani M, Casinovi CG, Cerrini S, Fedeli W, Pellicciari R, Santurbano B, Vaciago A. Experientia. 1968;24:631–635. - PubMed
-
- Barrow KD, Barton DHR, Chain EB, Ohnsorge UFW, Thomas R. Chem Commun. 1968:1198–1200.
-
- Ballio A, Casinovi CG, D'Alessio V, Grandolini G, Randazzo G, Rossi C. Experientia. 1974;30:844–845. - PubMed
-
- Barrow KD, Barton DHR, Chain E, Bageenda-Kasujja D, Mellows G. J Chem Soc Perkin Trans 1. 1975:877–883.
-
- Tuset JJ, Portilla T. Can J Bot. 1989;67:13275–13280.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

