Trigger Factor can antagonize both SecB and DnaK/DnaJ chaperone functions in Escherichia coli
- PMID: 17360615
- PMCID: PMC1805596
- DOI: 10.1073/pnas.0608232104
Trigger Factor can antagonize both SecB and DnaK/DnaJ chaperone functions in Escherichia coli
Abstract
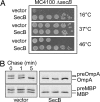
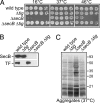
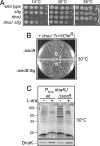
Polypeptides emerging from the ribosome are assisted by a pool of molecular chaperones and targeting factors, which enable them to efficiently partition as cytoplasmic, integral membrane, or exported proteins. In Escherichia coli, the chaperones SecB, Trigger Factor (TF), and DnaK are key players in this process. Here, we report that, as with dnaK or dnaJ mutants, a secB null strain exhibits a strong cold-sensitive (Cs) phenotype. Through suppressor analyses, we found that inactivating mutations in the tig gene encoding TF fully relieve both the Cs phenotype and protein aggregation observed in the absence of SecB. This antagonistic effect of TF depends on its ribosome-binding and chaperone activities but unrelated to its peptidyl-prolyl cis/trans isomerase (PPIase) activity. Furthermore, in contrast to the previously known synergistic action of TF and DnaK/DnaJ above 30 degrees C, a tig null mutation partially suppresses the Cs phenotype exhibited by a compromised DnaK/DnaJ chaperone machine. The antagonistic role of TF is further exemplified by the fact that the secB dnaJ double mutant is viable only in the absence of TF. Finally, we show that, in the absence of TF, more SecA and ribosomes are associated with the inner membrane, suggesting that the presence of TF directly or indirectly interferes with the process of cotranslational protein targeting to the Sec translocon.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Bukau B, Deuerling E, Pfund C, Craig EA. Cell. 2000;101:119–122. - PubMed
-
- Young JC, Agashe VR, Siegers K, Hartl FU. Nat Rev Mol Cell Biol. 2004;5:781–791. - PubMed
-
- Lill R, Crooke E, Guthrie B, Wickner W. Cell. 1988;54:1013–1018. - PubMed
-
- Luirink J, Sinning I. Biochim Biophys Acta. 2004;1694:17–35. - PubMed
-
- Veenendaal AK, van der Does C, Driessen AJ. Biochim Biophys Acta. 2004;1694:81–95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

