Concerted ATP-induced allosteric transitions in GroEL facilitate release of protein substrate domains in an all-or-none manner
- PMID: 17360617
- PMCID: PMC1805612
- DOI: 10.1073/pnas.0700070104
Concerted ATP-induced allosteric transitions in GroEL facilitate release of protein substrate domains in an all-or-none manner
Abstract
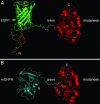
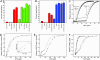
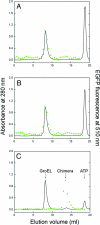
The double-ring chaperonin GroEL mediates protein folding, in conjunction with its helper protein GroES, by undergoing ATP-induced conformational changes that are concerted within each heptameric ring. Here we have examined whether the concerted nature of these transitions is responsible for protein substrate release in an all-or-none manner. Two chimeric substrates were designed, each with two different reporter activities that were recovered after denaturation in GroES-dependent and independent fashions, respectively. The refolding of the chimeras was monitored in the presence of GroEL variants that undergo ATP-induced intraring conformational changes that are either sequential (F44W/D155A) or concerted (F44W). Our results show that release of a protein substrate from GroEL in a domain-by-domain fashion is favored when the intraring allosteric transitions of GroEL are sequential and not concerted.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Concerted release of substrate domains from GroEL by ATP is demonstrated with FRET.J Mol Biol. 2008 Jul 18;380(4):717-25. doi: 10.1016/j.jmb.2008.05.021. Epub 2008 May 17. J Mol Biol. 2008. PMID: 18556021 Free PMC article.
-
A kinetic analysis of the nucleotide-induced allosteric transitions of GroEL.J Mol Biol. 1999 Oct 29;293(3):667-84. doi: 10.1006/jmbi.1999.3138. J Mol Biol. 1999. PMID: 10543958
-
Sequential ATP-induced allosteric transitions of the cytoplasmic chaperonin containing TCP-1 revealed by EM analysis.Nat Struct Mol Biol. 2005 Mar;12(3):233-7. doi: 10.1038/nsmb901. Epub 2005 Feb 6. Nat Struct Mol Biol. 2005. PMID: 15696173
-
Mechanism of substrate recognition by the chaperonin GroEL.Biochem Cell Biol. 2001;79(5):569-77. Biochem Cell Biol. 2001. PMID: 11716298 Review.
-
Structure and allostery of the chaperonin GroEL.J Mol Biol. 2013 May 13;425(9):1476-87. doi: 10.1016/j.jmb.2012.11.028. Epub 2012 Nov 24. J Mol Biol. 2013. PMID: 23183375 Review.
Cited by
-
GroEL and CCT are catalytic unfoldases mediating out-of-cage polypeptide refolding without ATP.Proc Natl Acad Sci U S A. 2013 Apr 30;110(18):7199-204. doi: 10.1073/pnas.1219867110. Epub 2013 Apr 12. Proc Natl Acad Sci U S A. 2013. PMID: 23584019 Free PMC article.
-
Folding and assembly defects of pyruvate dehydrogenase deficiency-related variants in the E1α subunit of the pyruvate dehydrogenase complex.Cell Mol Life Sci. 2018 Aug;75(16):3009-3026. doi: 10.1007/s00018-018-2775-2. Epub 2018 Feb 14. Cell Mol Life Sci. 2018. PMID: 29445841 Free PMC article.
-
Weak intra-ring allosteric communications of the archaeal chaperonin thermosome revealed by normal mode analysis.Biophys J. 2012 Sep 19;103(6):1285-95. doi: 10.1016/j.bpj.2012.07.049. Biophys J. 2012. PMID: 22995501 Free PMC article.
-
Concerted release of substrate domains from GroEL by ATP is demonstrated with FRET.J Mol Biol. 2008 Jul 18;380(4):717-25. doi: 10.1016/j.jmb.2008.05.021. Epub 2008 May 17. J Mol Biol. 2008. PMID: 18556021 Free PMC article.
-
Structural insight into the cooperation of chloroplast chaperonin subunits.BMC Biol. 2016 Apr 12;14:29. doi: 10.1186/s12915-016-0251-8. BMC Biol. 2016. PMID: 27072913 Free PMC article.
References
-
- Thirumalai D, Lorimer GH. Annu Rev Biophys Biomol Struct. 2001;30:245–269. - PubMed
-
- Saibil HR, Horwich AL, Fenton WA. Adv Protein Chem. 2002;59:45–72. - PubMed
-
- Horovitz A, Willison KR. Curr Opin Struct Biol. 2005;15:646–651. - PubMed
-
- Braig K, Otwinowski Z, Hegde R, Boisvert DC, Joachimiak A, Horwich AL, Sigler PB. Nature. 1994;371:578–586. - PubMed
-
- Hunt JF, Weaver AJ, Landry SJ, Gierasch L, Diesenhofer J. Nature. 1996;379:37–45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

