Concerted ATP-induced allosteric transitions in GroEL facilitate release of protein substrate domains in an all-or-none manner
- PMID: 17360617
- PMCID: PMC1805612
- DOI: 10.1073/pnas.0700070104
Concerted ATP-induced allosteric transitions in GroEL facilitate release of protein substrate domains in an all-or-none manner
Abstract
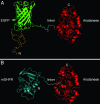
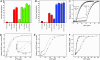
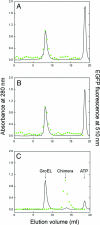
The double-ring chaperonin GroEL mediates protein folding, in conjunction with its helper protein GroES, by undergoing ATP-induced conformational changes that are concerted within each heptameric ring. Here we have examined whether the concerted nature of these transitions is responsible for protein substrate release in an all-or-none manner. Two chimeric substrates were designed, each with two different reporter activities that were recovered after denaturation in GroES-dependent and independent fashions, respectively. The refolding of the chimeras was monitored in the presence of GroEL variants that undergo ATP-induced intraring conformational changes that are either sequential (F44W/D155A) or concerted (F44W). Our results show that release of a protein substrate from GroEL in a domain-by-domain fashion is favored when the intraring allosteric transitions of GroEL are sequential and not concerted.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Thirumalai D, Lorimer GH. Annu Rev Biophys Biomol Struct. 2001;30:245–269. - PubMed
-
- Saibil HR, Horwich AL, Fenton WA. Adv Protein Chem. 2002;59:45–72. - PubMed
-
- Horovitz A, Willison KR. Curr Opin Struct Biol. 2005;15:646–651. - PubMed
-
- Braig K, Otwinowski Z, Hegde R, Boisvert DC, Joachimiak A, Horwich AL, Sigler PB. Nature. 1994;371:578–586. - PubMed
-
- Hunt JF, Weaver AJ, Landry SJ, Gierasch L, Diesenhofer J. Nature. 1996;379:37–45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

