Each rhodopsin molecule binds its own arrestin
- PMID: 17360618
- PMCID: PMC1805568
- DOI: 10.1073/pnas.0610886104
Each rhodopsin molecule binds its own arrestin
Abstract
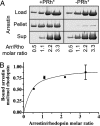
Arrestins (Arrs) are ubiquitous regulators of the most numerous family of signaling proteins, G protein-coupled receptors. Two models of the Arr-receptor interaction have been proposed: the binding of one Arr to an individual receptor or to two receptors in a dimer. To determine the binding stoichiometry in vivo, we used rod photoreceptors where rhodopsin (Rh) and Arr are expressed at comparably high levels and where Arr localization in the light is determined by its binding to activated Rh. Genetic manipulation of the expression of both proteins shows that the maximum amount of Arr that moves to the Rh-containing compartment exceeds 80%, but not 100%, of the molar amount of Rh present. In vitro experiments with purified proteins confirm that Arr "saturates" Rh at a 1:1 ratio. Thus, a single Rh molecule is necessary and sufficient to bind Arr. Remarkable structural conservation among receptors and Arrs strongly suggests that all Arr subtypes bind individual molecules of their cognate receptors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- DK58404/DK/NIDDK NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- P30 HD015052/HD/NICHD NIH HHS/United States
- NS45117/NS/NINDS NIH HHS/United States
- EY11500/EY/NEI NIH HHS/United States
- EY08126/EY/NEI NIH HHS/United States
- P30 EY008126/EY/NEI NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- R01 NS045117/NS/NINDS NIH HHS/United States
- HD15052/HD/NICHD NIH HHS/United States
- DK20593/DK/NIDDK NIH HHS/United States
- R01 EY011500/EY/NEI NIH HHS/United States
- CA68485/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

