Regulation of a glutamyl-tRNA synthetase by the heme status
- PMID: 17360620
- PMCID: PMC1805545
- DOI: 10.1073/pnas.0611611104
Regulation of a glutamyl-tRNA synthetase by the heme status
Abstract
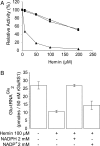
Glutamyl-tRNA (Glu-tRNA), formed by Glu-tRNA synthetase (GluRS), is a substrate for protein biosynthesis and tetrapyrrole formation by the C(5) pathway. In this route Glu-tRNA is transformed to delta-aminolevulinic acid, the universal precursor of tetrapyrroles (e.g., heme and chlorophyll) by the action of Glu-tRNA reductase (GluTR) and glutamate semialdehyde aminotransferase. GluTR is a target of feedback regulation by heme. In Acidithiobacillus ferrooxidans, an acidophilic bacterium that expresses two GluRSs (GluRS1 and GluRS2) with different tRNA specificity, the intracellular heme level varies depending on growth conditions. Under high heme requirement for respiration increased levels of GluRS and GluTR are observed. Strikingly, when intracellular heme is in excess, the cells respond by a dramatic decrease of GluRS activity and the level of GluTR. The recombinant GluRS1 enzyme is inhibited in vitro by hemin, but NADPH restores its activity. These results suggest that GluRS plays a major role in regulating the cellular level of heme.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Ibba M, Söll D. Genes Dev. 2004;8:731–738. - PubMed
-
- Park SG, Ewalt KL, Kim S. Trends Biochem Sci. 2005;30:569–574. - PubMed
-
- Ryckelynck M, Giege R, Frugier M. Biochimie. 2005;87:835–845. - PubMed
-
- Feng L, Tumbula-Hansen D, Min B, Namgoong S, Salazar J, Orellana O, Söll D. In: Aminoacyl-tRNA Synthetases. Ibba M, Cusack S, editors. Georgetown, TX: Landes; 2005. pp. 314–319.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

