Host shutoff during productive Epstein-Barr virus infection is mediated by BGLF5 and may contribute to immune evasion
- PMID: 17360652
- PMCID: PMC1805610
- DOI: 10.1073/pnas.0611128104
Host shutoff during productive Epstein-Barr virus infection is mediated by BGLF5 and may contribute to immune evasion
Abstract
Relatively little is known about immune evasion during the productive phase of infection by the gamma(1)-herpesvirus Epstein-Barr virus (EBV). The use of a unique system to isolate cells in lytic cycle allowed us to identify a host shutoff function operating in productively EBV-infected B cells. This impairment of protein synthesis results from mRNA degradation induced upon expression of the early lytic-cycle gene product BGLF5. Recently, a gamma(2)-herpesvirus, Kaposi sarcoma herpesvirus, has also been shown to encode a host shutoff function, indicating that host shutoff appears to be a general feature of gamma-herpesviruses. One of the consequences of host shutoff is a block in the synthesis of HLA class I and II molecules, reflected by reduced levels of these antigen-presenting complexes at the surface of cells in EBV lytic cycle. This effect could lead to escape from T cell recognition and elimination of EBV-producing cells, thereby allowing generation of viral progeny in the face of memory T cell responses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
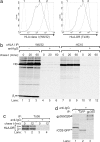
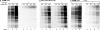
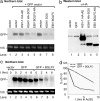
References
-
- Rickinson AB, Kieff E. In: Fields Virology. Knipe DM, Howley PM, editors. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 2575–2627.
-
- Yao QY, Rickinson AB, Epstein MA. Int J Cancer. 1985;35:35–42. - PubMed
-
- Roizman B, Pellet PE. In: Fields Virology. Knipe DM, Howley PM, editors. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 2381–2397.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

